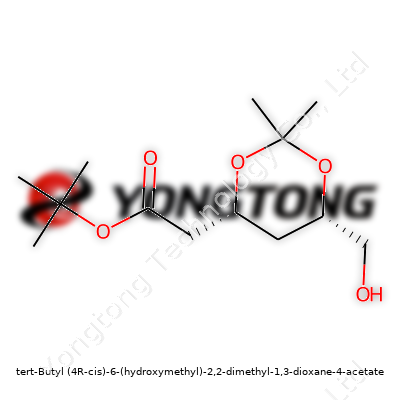
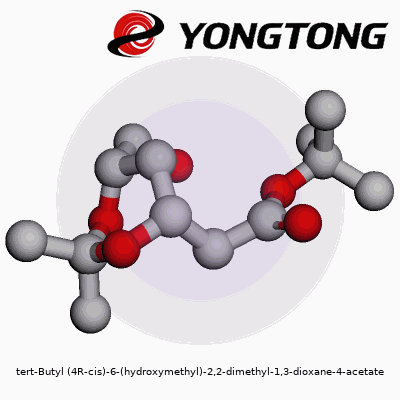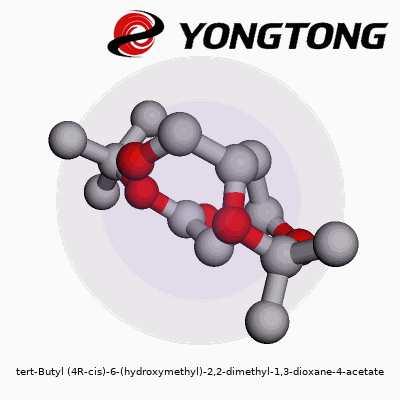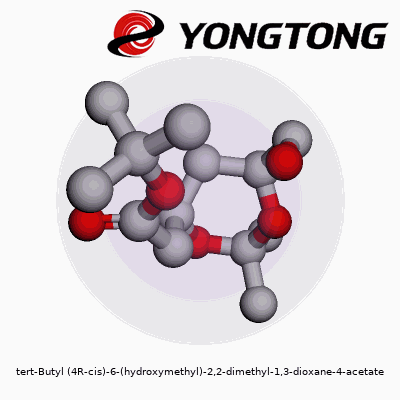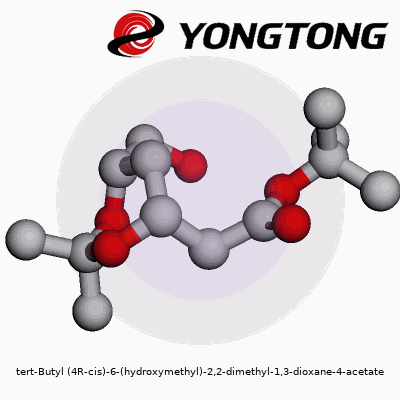
tert-Butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate: A Deep Dive
Historical Development
Chemical research always brings together a mix of curiosity, trial and error, and gradual understanding. With compounds like tert-Butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate, this process stretches over decades of organic chemistry innovation. Early dioxane derivatives emerged from work in carbohydrate and protecting group chemistry during the 1960s and 1970s. Chemists, needing ways to mask reactive alcohol groups, relied on acetonides and dioxanes. The addition of bulky tert-butyl and methyl groups pushed development toward molecules with greater steric protection and stability. Strong demand in pharmaceutical synthesis—searching for efficient and selective intermediates—kept interest high. Universities and specialty labs contributed data by publishing synthetic approaches and reactivity profiles, focusing on selectivity and mild reaction conditions. Over time, improvements in chiral catalysis and selective oxidation allowed for greater control over stereochemistry, enabling the isolation of isomers like the (4R-cis) configuration. This legacy continues to shape new applications and inspire creative modifications in both academic and industrial settings.
Product Overview
tert-Butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate serves as a multi-purpose intermediate. Its core structure—a dioxane ring—offers a balance of rigidity and reactivity, especially appealing in the synthesis of complex molecules. The addition of a tert-butyl ester and dimethyl substitutions influences not just reactivity, but also physical handling. This combination makes it attractive where chemoselectivity and mild deprotection matter. It appears widely in research settings, pharmaceutical labs, and fine chemical manufacturing, supporting the endless march toward more selective and efficient syntheses.
Physical & Chemical Properties
Compounds like this one show up as white to off-white solids at room temperature, sometimes forming crystalline powders. The structure blocks water absorption by the core ring, keeping the compound stable under normal storage. Flashpoint ranges above 100°C limit flammability risks in most labs, but the tert-butyl and acetate groups make it susceptible to acid-catalyzed hydrolysis under strong conditions. Its melting point, usually above 50°C, facilitates purification by recrystallization or chromatography. Chemical reactivity focuses on the exposed hydroxymethyl group and ester link—these enable further functionalization, reduction, or hydrolysis, setting the stage for downstream chemistry. Spectral properties, including characteristic IR carbonyl stretches around 1740 cm⁻¹, cluster of methyl proton signals in ¹H NMR, and distinct ring carbon peaks in ¹³C NMR, provide fingerprints for quality control and batch verification.
Technical Specifications & Labeling
Standard technical data cover purity, moisture content, residual solvents, and chiral ratio, often verified through HPLC, GC-MS, and NMR. Purity above 98% is typical for research and drug synthesis. Residual solvent content gets a close look, as impurities impact both safety and effectiveness in downstream reactions. Packaging usually protects against light and moisture, using amber glass or HDPE bottles with tamper-proof seals. Labels keep things clear: compound name, structure diagram, batch number, manufacture and expiry date, recommended storage (cool, dry, below 25°C), and safety warnings. For regulatory compliance, supplier labels must list country of origin, operator details, and test results from an in-house COA. Users must check certificates for batch-specific data before starting sensitive or high-value work.
Preparation Method
Synthesis routes for tert-Butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate often start with a protected diol. Chemists kick things off with selective acetonide formation, using acid catalysis and controlled temperature to coax desired rigidity and stereochemistry from the sugar-like backbone. Installation of methyl groups may come via Grignard addition or alkyl lithium reagents, giving that crucial dimethyl block at C2. The hydroxymethyl branch usually emerges from reduction of an ester, aldehyde, or protected alcohol—controlled sodium borohydride reduction stands out for its selectivity. Forming the tert-butyl acetate, a Steglich-type esterification builds the necessary linkage at the C4 ring site. Stereochemical purity at the (4R-cis) position relies on starting materials and chiral auxiliaries. At scale, activated molecular sieves, slow reagent addition, and low reaction temperatures keep side-products at bay. End-game workup runs through neutralization, organic extraction, rotary evaporation, and repeated crystallization, all aimed at batch-to-batch reproducibility—a lesson learned after years of wrangling with problem reactions.
Chemical Reactions & Modifications
The acetate and tert-butyl protection open up deprotection strategies. Mild acid or base hydrolysis clips off the tert-butyl group, revealing a free carboxylic acid ready for amide coupling, peptide extension, or further acylation. The dioxane ring stands up well under neutral and mildly basic conditions, letting selective reactions target exposed groups. The hydroxymethyl arm supports oxidation to aldehydes or carboxylic acids using TEMPO, PCC, or Swern methods. Advanced methodologies, like cross-coupling or azide substitution, piggyback on the freed alcohol, building in new carbon linkages, azides, or other pharmacophores. The rigid ring shields sensitive sites, preventing unwanted overreaction or racemization. Intramolecular cyclization or ring opening creates access to exotic analogs and derivatives, giving medicinal chemists or process engineers flexible starting points for thousands of new candidates or scale-ups. Plenty of researchers prefer these robust intermediates for combinatorial chemistry, fragment-based design, or pilot plant optimization, citing years of experience battling less stable or too-reactive alternatives.
Synonyms & Product Names
Chemists rarely stick to one name for a compound—old habits, supplier quirks, and shorthand all play a part. Journals and catalogs list alternative descriptors like “tert-butyl cis-4-acetoxy-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane” or “6-Hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester”. Some literature opts for “dioxane-acetate tert-butyl ester”, dropping chiral descriptors. Specialty suppliers invent house abbreviations for cataloging, prompting double-checks against structure diagrams. Trade names might reference stability features, application focus, or batch modifications, complicating ordering and regulatory documentation. Checking both CAS numbers and full systematic names keeps confusion and costly misorders at bay.
Safety & Operational Standards
Managing chemical hazards goes beyond ticking regulatory boxes. Handling tert-butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate means using splash goggles, nitrile gloves, and lab coats, plus running reactions under fume hoods. Recrystallization solvents—often ethyl acetate, hexanes, or isopropanol—bring flammability and inhalation hazards. Acids for deprotection or esters for coupling demand quick neutralization and careful waste disposal. Safety data sheets spell out eye and skin irritancy, long-term exposure data, and reactivity with strong acids or oxidizers. Good training keeps weaker colleagues or students out of harm’s way—a lesson drummed in by tight deadlines and the odd accident. Emergency procedures require eyewash stations, safety showers, and access to first-aid. Disposal routes follow both local and international rules—segregating halogenated waste, using spill pads, and tracking batch numbers for regulatory inspection. Contamination checks on workspaces cut the risk of accidental cross-reaction, product loss, or even trace contamination of pharmaceuticals where parts-per-million really matters.
Application Area
This compound’s core role lands squarely in the pharmaceutical sector, where synthetic intermediates shape the path to active pharmaceutical ingredients. Functional protection, selective modification, and easy deprotection simplify the route to complex stereocenters. Kilogram-scale batch production enables pilot plant scale-up for late-stage drug candidates—critical for progressing from early discovery to the clinic. Outside drugs, the compound serves as a building block for agrochemicals, custom specialty polymers, and advanced materials. In academic circles, its robust structure and versatile chemistry get frequent mention in peer-reviewed publications, where chemists map out new synthetic routes, ring-opening pathways, or dual-protection strategies. Startup companies seeking patent space on innovative process methods often deploy this dioxane to sharpen their edge against competitors. Some biomedical researchers even adapt it for conjugation studies, using its protected groups to anchor fluorescent labels or create prodrugs with tailored release mechanisms.
Research & Development
Laboratories and R&D teams tinker with both the parent compound and its variants to push boundaries. Chiral separation, protecting group compatibility, and greener synthetic pathways drive much of the recent push. Flow chemistry tools come into play to improve batch reproducibility, slash solvent use, and lower energy costs. Screening new catalysts to shorten reaction steps has become the norm, with “one-pot” transformations reducing hands-on time and hazardous reagent exposure. Spectroscopists keep looking for robust QC signals—lowering the cost and uncertainty of scaling from milligrams to kilos. Published data shows academic and corporate partnerships speeding up progress, as open communication unlocks lessons learned by both sides. In my own work, access to reliable, stereochemically pure intermediates made iterative route design faster, freeing time for hypothesis testing instead of problem-solving missteps or chasing down unknown impurities. National and industry grants now earmark funding for improved synthesis of high-value intermediates, seeing the connection between cost, waste, and regulatory hurdles in commercial drug launches.
Toxicity Research
Testing toxicity isn’t just paperwork—it’s essential. tert-Butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate draws attention because its fragments—tert-butyl ester, acetate, dioxane—each bring unique toxicological profiles. Acute effects, like irritation on skin or eyes, tend to resolve after rinsing, but long-term animal studies explore oral, inhalation, and dermal effects. Published data point to low acute systemic toxicity at lab exposure levels, yet breakdown products sometimes trigger concern as potential alkylators, especially in aquatic environments. Regulatory agencies scrutinize batch-specific impurities because certain byproducts, left unchecked, could create risks or regulatory headaches. Chronic exposure studies influence storage guidelines, handling protocols, and maximum daily exposure calculations for pharmaceutical APIs. Looking at the bigger picture, responsible labs invest in detailed risk assessments and personal monitoring systems, both to satisfy auditors and to take care of teams.
Future Prospects
Looking ahead, research into tert-Butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate isn’t slowing down. Shifts in green chemistry and process intensification might cut waste and scale cost barriers, making this and similar intermediates more available for both blockbuster drugs and orphan treatments. AI-driven route design and predictive analytics are likely to bring down optimization time, reshaping how chemists approach synthesis planning. Regulatory changes keep steering process design toward fewer hazardous reagents and more recyclable solvents, which could prompt broader use of non-traditional protection groups. In practice, success in these areas means not just better yields or lower costs, but safer workplaces and faster regulatory clearance for new medications. With every new isotopically labeled analog or stereospecific route, chances for deeper biomedical and materials science application grow—bridging gaps between idea and reality, discovery and delivery. Over the coming years, both academic breakthroughs and industrial implementation are likely to keep this family of compounds at the front of chemical innovation.
Digging Into the Skeleton: What's Behind the Name?
Molecules carry stories in their names. Tert-butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate might look like a tongue twister, but every part hints at the design. Picture a six-membered ring—named 1,3-dioxane—with two oxygen atoms and a backbone dotted by methyl groups at the 2-position. At the fourth carbon, the acetate function grabs attention, attached through an ester linkage. This ring isn’t flat; its three-dimensional twist, locked in the (4R-cis) orientation, defines how it bumps up against neighboring molecules.
The tert-butyl attachment and the two methyl groups keep the ring shielded and notoriously unreactive, except at sites tailored for chemistry—like the hydroxymethyl group pointing out from the sixth carbon. Experience from many years in a synthetic organic lab has taught me how these groups protect sensitive parts of a molecule as the rest of the structure endures tough reaction conditions.
Why Chemists Care About Its Shape
This structure pops up as an intermediate in the worlds of drug discovery and synthetic methodology. The rigid dioxane ring—bolstered by bulky tert-butyl and methyl groups—offers a scaffold that resists attack from acids, bases, or even water. Chemists often build molecules piece by piece, needing ways to shield fragile spots. If you've ever spent time puzzling over reaction failures, the presence of a robust ring like this makes all the difference.
The placement of oxygens and the acetate arm allow selective reactions. An acetoxy group is famous for its ability to act as a leaving group—paving a route for new carbon-carbon or carbon-heteroatom bonds. The dioxane ring keeps everything in a known orientation, which matters when a medicine’s effect comes down to which way one atom faces.
Chemical Structure: Drawing It Out with Words
Think of this molecule like a bicycle wheel with two spokes made of oxygen. At the hub (carbon four), the road forks: one path leads to the acetate, the other to the main wheel. On the rim, two plump methyl cushions append at carbon two, while the sixth point supports the hydroxymethyl post, jutting out like a handle. The tert-butyl cap hangs near the ester, bulking up one side.
If you’ve ever mentally built molecules while planning a synthesis, you know spatial orientation rules the game. Here, the (4R-cis) bit blocks one face of the molecule, making it possible to steer reactions away from the wrong place, which is priceless in complex syntheses.
Why It Matters: Beyond Academic Curiosity
As research focuses more on targeted therapies and intelligent drug candidates, compounds like tert-butyl (4R-cis)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxane-4-acetate matter for practical reasons. Their architecture gives both stability and fine control over chemical transformations. Mistaking a configuration—or ignoring the role of a protective group—can sink weeks of effort or muddle test results.
Going forward, the push for greener, faster, and more predictable chemistry probably leans even harder on such robust cyclic protecting groups. Smart design up front leads to fewer purification steps, cleaner reactions, and less waste. The fine print in a structure often shapes the success of modern chemistry.
Household Staples and Daily Living
Growing up, I always found a blue box of baking soda tucked in the fridge. Sodium bicarbonate, better known as baking soda, is that humble compound with long roots in kitchens across the world. Families use it to neutralize fridge odors, polish old pans, and whip up batch after batch of cookies. It’s not just for baking; plenty of people turn it into a makeshift toothpaste or a soothing bath soak. According to the American Cleaning Institute, over 80% of U.S. households keep baking soda handy for cleaning and deodorizing jobs.
Healthcare and Well-Being
Doctors and nurses rely on sodium bicarbonate inside hospital walls just as much as families do at home. Emergency rooms stock it for patients whose blood turns too acidic, often in cases of kidney issues or uncontrolled diabetes. It’s a quick, reliable buffer that helps stabilize things fast. Dentists point out its gentle abrasive quality, helping remove plaque without being harsh. In my own life, a baking soda paste knocked out the sting of a bee bite quicker than any store-bought cream ever did.
Food and Beverage Manufacturing
Food manufacturers have turned to sodium bicarbonate to keep doughs light and crispy. Whether it’s fluffy pancakes or crunchy pretzels, this compound reacts with acids in recipes, releasing bubbles that make baked goods rise. Brewers use it to tweak water chemistry, making sure beer maintains its signature flavor profile. The Center for Science in the Public Interest highlights how widespread leavening agents like this are, noting a massive impact on the texture and shelf life of everything from bread to crackers.
Industrial and Environmental Roles
Factories count on sodium bicarbonate to tackle pollution before it ever reaches the sky. Flue gas treatment systems inject the powder to capture sulfur dioxide and other pollutants, making power plants cleaner. In the textile industry, it preps fibers for dyeing so colors stay true. Water treatment facilities use it to nudge pH levels in the right direction, preventing pipeline corrosion. My neighbor, who works for the local wastewater plant, once pointed out how they measure baking soda by the ton, all for the sake of balancing city water.
Personal Care and Hygiene
The beauty aisle is packed with products that mention baking soda on the label—deodorants, face scrubs, dry shampoos. The logic feels timeless: it absorbs smells and gently buff away grime. Dermatologists advise moderation, warning it’s alkaline, but its popularity doesn’t seem to fade. Grandparents trusted a bit of baking soda in bathwater to soothe itchy skin, knowledge passed down long before branded baby powders and lotions ever came along.
Fire Safety and Emergency Use
Small kitchen fires can get out of hand fast. Tossing a handful of baking soda over a grease fire chokes off the flames, a trick taught in many fire safety workshops. The National Fire Protection Association recommends keeping some near the stove as an extra layer of protection, especially in homes without easy access to a fire extinguisher.
Looking Toward Better Solutions
Sodium bicarbonate’s legacy stretches from cleaning floors to balancing blood. Its low cost and gentle impact on health and the environment convince many to reach for it before heavier chemicals. Food producers and factories could continue shifting toward it, phasing out harsher options. Research teams test new medical uses, exploring if it can help manage kidney disease long term. People trust this simple powder because it works, generation after generation.
Why Purity Matters in Everyday Use
Anyone who has worked in a lab or managed inventory for production knows that ingredient purity isn’t just a technical specification. The impact shows up in every result—good or bad. Impurities sneak in, and suddenly your analysis jumps all over the place, or a final product gets flagged for rework. I’ve found that aiming for high-purity batches, above 99% if possible, can save a lot of future headaches. It lets you trust your results. Customers rely on this consistency, whether they’re ordering for medicine prep, food manufacturing, or industrial work. High purity means fewer unknowns and less troubleshooting.
Take pharmaceuticals as an example. The FDA and other regulators don’t tolerate contaminants above tiny levels. A small impurity in one lot can ruin months of hard work and shrink profit margins just as fast. In food production, off-flavors and safety risks grow when purity isn’t front and center. Whether regulations call for pharmaceutical grade or technical grade, sourcing top-quality supplies shows genuine care for customer safety and business reputation.
Everyday Habits for the Right Storage
Storage rules sound simple—keep it cool, keep it dry—but missing small steps leads to real loss. I once managed a storeroom where humidity jumped, and an entire shelf of ingredients clumped and turned useless. Good storage starts with a clean, organized space. Temperatures between 2°C and 8°C work for many sensitive chemicals, medicine intermediates, and flavors. Food-grade materials last longer in sealed containers, away from sunlight. Too much moisture and temperature swings make powders cake and liquids separate.
Labeling is a small detail that pays off. Clear dates, batch numbers, and open/close tracking cuts down accidental mix-ups. In less controlled rooms, having a backup fridge or climate-controlled cabinet prevents big losses during power outages or hot seasons. Even non-sensitive supplies like salts and solvents hold up better away from air and light.
Cross-contamination still trips up a lot of teams. I’ve seen otherwise careful technicians set uncleaned scoops right back in storage bins. Using color-coded tools and regular cleaning checks keeps supply rooms from becoming a guessing game. Closed containers and single-use tools might look like overkill, but they stop serious headaches later—especially with ingredients that react to air or humidity.
Building Daily Trust Through Attention to Detail
Customers expect reliability. That’s why suppliers and labs who take purity and storage seriously earn trust, repeat orders, and good word-of-mouth. Anyone new to quality management learns fast: shortcuts today turn into support calls and warranty claims tomorrow. Paper trails, strict supplier checks, and batch-tested samples back up claims about purity.
Digital tracking makes compliance audits less painful. Simple barcoding and temperature loggers flag problems early. Even for small businesses, spending the extra time to store and handle products correctly makes reputations stronger. Teams that go beyond minimum specs become the folks everyone trusts for clean, safe, high-quality goods.
Purity and storage habits don’t just meet requirements—they help people do their best work and deliver the kind of reliability that builds lasting partnerships. By treating each batch as the lifeline of someone’s final product, businesses avoid waste and guarantee results everyone can stand behind.
Why Sourcing Size Matters
People in manufacturing, biotech, or even small research labs often hit a wall over compound availability. The answer to a basic question—can we buy it in bulk, or are we stuck with small vials from specialty suppliers?—sometimes shapes the entire project. Getting a few grams for a science demo at home isn’t the same as needing 15 kilograms for a commercial run. That difference, though, ties directly into cost, safety, and sometimes progress itself.
A decade back, running those chemistry projects in a university lab, I remember the grind. Some compounds were everywhere, others felt like secrets. Occasionally, we ended up diluting focus, swapping out reactions, just because nobody sold the thing we needed in buckets. There’s a direct line from research success to what’s actually on the shelf. Amazon can deliver books overnight, but specialty chemicals ride a snail.
What Holds Producers Back?
Suppliers balance risk. They keep stocks of what turns over fast. Explosive growth in industries like green tech or pharmaceuticals means more of these niche compounds should reach bulk status. But until enough people demand a material, most manufacturers won't fire up their big reactors. It shocks some to learn many compounds get made only once a year, just enough to fill little glass bottles for research orders.
Volume drives price. It’s not just a bigger bottle—bulk supply takes heavier logistics, quality documentation, and regulatory steps. Small stock might come from repacked batches, sometimes skimmed off leftovers from industrial production. That’s cost-efficient for producers, but it frustrates groups who want a big, consistent batch for scaling up trials or making a product.
Hidden Costs of Small-Batch Only
Small-scale purchase looks easy. Just order a bottle, pay online, job done. Except, most of the time, per-gram costs hit the roof. A kilo purchased that way can cost ten times what bulk buyers pay. Worse, jumping suppliers by necessity can bring inconsistent product quality, creating new headaches.
This reality holds back innovation in small companies. You may have a prototype that stuns at the bench, but good luck wooing investors if you say your core ingredient only shows up in 25-gram lots. That message kills more projects than a failed feasibility study.
Better Solutions Exist
Transparency from suppliers offers a real fix. Listing supply capability ahead of price, instead of a bland “inquire for larger amounts,” makes life easier for busy researchers and startup owners. Long-term, collective buying groups could shift the dynamic—labs band together, cut deals, and create new demand. Direct partnerships between buyers and custom synthesis labs help too. Sometimes, it only takes a modest volume commitment to flip the switch from novelty to bulk.
Open discussions about compound availability—both for small and bulk users—open doors for everyone. Information flows, people plan better, money and time get saved. If more suppliers share honest information about bulk supply options, and if buyers signal their needs early, more companies and researchers can focus on solving bigger problems, not hunting chemicals.
Not All Chemicals Get along with Carelessness
Most folks don’t spend their days thinking about chemical safety, until something goes sideways. I learned this the hard way in my first year at a summer factory job, prying open a bag of powder without reading the label. A headache and some irritated skin later, I realized those warnings have a point. Even those of us not working in a research lab or huge plant can cross paths with chemicals at home, in the garage, or in yard work. A label tells a story, and it's one worth listening to.
Protective Gear Goes Farther Than You Think
Gloves and goggles look a little silly until you remember how much one chemical splash can change. Proper safety glasses can keep acid from blinding you. Nitrile gloves keep solvents off the hands. I once watched a co-worker ignore a mask while cleaning with ammonium-based sprays. His cough stuck around for weeks. A few dollars spent at the hardware store beats seeing a doctor. It’s smart to keep a stash of gloves, glasses, and masks if you're often handling anything stronger than vinegar.
Ventilation Matters More Than You’d Expect
Open windows make a difference, even if the task seems simple. Solvents, bleaches, acids, and strong bases send fumes into the air, and those fumes linger. More than once I’ve smelled metal cleaners hanging in the air long after the rag got thrown out. Chemical exposure doesn’t always hit fast. Headaches, fatigue, or skin irritation can show up hours later. Sometimes symptoms get mistaken for flu or a bad day. Fans work, but nothing beats a cross breeze from windows or an exhaust fan.
Storage and Labeling: Not Just for the Organized
It’s easy to forget which bottle holds what, until a faded label or sun-bleached symbol ends up causing a mix-up. My grandfather kept an old paint thinner jug on his shelf filled with something else. No one knew what it was, so everyone just avoided it. That’s risky. Chemicals react in dangerous ways—mixing bleach and ammonia turns the air poisonous in seconds. Store acids separate from bases, keep fuels away from anything flammable, and never let kids or pets near these cupboards. Read the label on refills and leftovers, don’t just trust your memory.
Emergency Plans Save Lives
We talk a lot about “what to do if” after something has already gone wrong. Knowing exactly where to find an eyewash station or fire extinguisher gives confidence and shaves off precious seconds. I keep baking soda within arm’s reach when working with acids. Keeping poison control numbers on the fridge isn’t only for young families. Neighbors can help each other, too—sharing tips and stories, building a culture where safety isn’t just for “professionals.” It becomes a habit.
Education Over Fear
Worry won't keep you safe, but paying attention will. Training matters in factories, warehouses, and even garages. Teaching new folks about what chemicals do and don’t mix, how storage works, and what protective steps look like prevents disasters before they start. Asking questions, reading up online, or taking a short class helps more than most people realize. Safety doesn’t ruin a job or hobby—it keeps everyone healthy enough to enjoy the next day, too.
Stay aware, stay prepared, and never underestimate what a label can teach you.