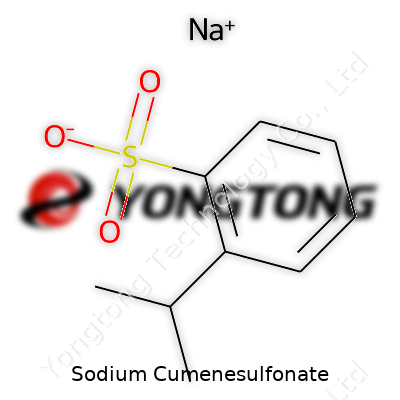
Sodium Cumenesulfonate: An In-Depth Commentary
Historical Development
Long before laundry detergents promised 99.9% stain removal, chemists had their heads down, finding ways to help water dissolve dirt and fats. Sodium cumenesulfonate emerged in the early half of the 20th century, right as the shift from soap to synthetic detergents picked up steam. Petroleum refining and sulfonation gained ground after World War II, letting researchers build aromatic sulfonates like sodium cumenesulfonate from cumene, a material made mainly by alkylating benzene with propylene. After more traditional alkylbenzene sulfonates hit the commercial market, sodium cumenesulfonate showed up as a supporting ingredient—boosting the performance of surfactant blends. Companies in North America, Europe, Japan, and later China, saw steady demand from cleaners, textile processing, and drilling fluids, all depending on sodium cumenesulfonate as a hydrotrope. By the mid-1960s, production facilities in the United States and Germany ran continuous sulfonation units. Even after stricter regulations on benzene and aromatic hydrocarbons pushed for safer chemicals, sodium cumenesulfonate never disappeared. Experience tells me chemistry never stands still, but certain building block molecules, for all their flaws, stick around because solutions still rely on them.
Product Overview
Walk into a warehouse stocked with specialty chemicals, and you’ll likely find bags or barrels stamped with alternate names for sodium cumenesulfonate, perhaps “sodium isopropylbenzenesulfonate”. Most folk in the industry recognize it as a colorless to pale yellow powder or sometimes a very pale liquid, always easy to handle and blend. Unlike some surfactants with sharp odors, sodium cumenesulfonate keeps its smell faint, almost imperceptible. It works as a hydrotrope, breaking the tension between water and greasy soils—making it essential for soap makers and floor-care chemists. Its presence in everything from metalworking fluids to dishwashing gels comes down to how it lets formulators get more out of less—letting less surfactant go further, especially in hard water.
Physical & Chemical Properties
Through years on the shop floor and in the lab, I've watched sodium cumenesulfonate bring predictable results. This sodium salt dissolves freely in water, forming a clear solution. Its melting point sits high enough that it hardly ever crystallizes in storage, always ready to go right from the hopper. The molecular structure centers around a benzene ring with a sulfonate group and an isopropyl group. This gives sodium cumenesulfonate a modest molar mass, enough bulk to stabilize emulsions but not so heavy that it weighs down fluid applications. Its pH in solution leans neutral to slightly alkaline, so formulators don't keep bottles of acid or base on standby just to balance it. Product consistency matters more than just numbers on a spec sheet—small variations in sodium cumenesulfonate rarely disturb finished goods, unlike some competitors that foam unexpectedly or produce odor.
Technical Specifications & Labeling
Labels on sacks of sodium cumenesulfonate don’t just carry a string of chemical names. Most suppliers include assay percentages—usually above 95%—with the rest being residual moisture and trace organic salts. Granulometry and loss on drying get tracked, too. For blends and liquids, viscosity and density appear as well, matched to each production batch. Chemists want this data to keep dosing precise, but tech staff on the floor just want to know it won't clog feeders or spill uncontrollably. Product labels list batch numbers, dates, and contact info for traceability, essential during recalls or audits. Labels also bear hazard pictograms (often the exclamation mark for irritants) and plain safety warnings. Having spent time reviewing our own SDS pages, I know strict standards help avoid ambiguity and blame games when things go wrong.
Preparation Method
Most of today’s sodium cumenesulfonate starts as cumene, produced through alkylation of benzene with propylene in a Friedel-Crafts catalyzed reaction, often with acidic catalysts like aluminum chloride. The cumene then reacts with sulfur trioxide in a controlled sulfonation, yielding cumenesulfonic acid. Neutralization with sodium hydroxide follows, forming the sodium salt ready for drying and standardization. Sulfonation control plays a real role—too much heat or too strong a reaction pushes impurities or scorched product, and yield drops. Neutralizing at the right temperature and rate prevents clumps and ensures uniform quality. Long hours learning process controls have shown how much raw material quality and reaction conditions shape the final product—lessons no spec sheet will ever spell out.
Chemical Reactions & Modifications
Chemists find sodium cumenesulfonate appealing because it resists oxidation and reduction under moderate conditions, maintaining stability in alkaline and neutral solutions. This anion swaps out its sodium easily for other cations, broadening its applications in different blend systems. Under strong heat or concentrated acid, the organic portion breaks down, releasing volatile organics and sulfonic acids. Lab research explores modifications—like swapping the alkyl side chain or converting to other salts—to tweak solubility, foaming, or dispersant qualities. Over the years, such research shaped how the product fits into not only detergents but cement additives, oil recovery chemicals, and bio-enzymatic cleaners.
Synonyms & Product Names
Ask three technical sales representatives about sodium cumenesulfonate, and you'll probably collect a handful of commercial names: “Sodium isopropylbenzenesulfonate”, “SCS”, “Kumensulfonate sodium salt”, along with manufacturer codes like “Hydrotrope S-15”. Old industry catalogs list it as Kumensulfonic acid, sodium salt. In the global market, some suppliers lean heavily on house brands, but the fundamental chemistry stays constant, only with minor tweaks in purity or particle size for niche buyers.
Safety & Operational Standards
No chemical ever comes without its share of safety talk. While not notorious for acute toxicity, sodium cumenesulfonate still features on irritant lists—proper gloves and goggles spare plant workers from rashes. Dust can trigger mild respiratory discomfort, especially in tight storage areas. Spill protocols focus more on slip hazards than fire, as it doesn't burn under ordinary circumstances. Environmental regulations guide proper neutralization and treatment in wastewater—especially important near streams and lakes prone to surfactant accumulation. I’ve seen the aftermath when such runoff gets ignored: foam collects in drainage ditches, and neighbors file complaints. Regular audits, hazard communication training, and enforced spill control do more than protect workers—they preserve the company’s right to stay in business.
Application Area
Few other hydrotropes see the range of use that sodium cumenesulfonate earns. Cleaning chemists count on it to help detergents perform at lower surfactant concentration, especially in hard water. Textile factories keep barrels on hand to boost dye solubility, while oilfield crews drench it into well drilling fluids to keep solids suspended and pumps unclogged. In concrete admixes, sodium cumenesulfonate acts as a dispersant, which leads to smoother pours and less water use. Dishwashing liquid and industrial cleaner formulas rely on it to help tricky ingredients like solvents and builders come together in stable solutions. My own experience repeats the story: customers don’t want novel chemistry for its own sake—they want performance that’s reliable in hot, cold, soft, or hard water.
Research & Development
Laboratories and R&D teams keep pushing the boundaries of what sodium cumenesulfonate can offer, often chasing more sustainable variants and performance at lower dosage. Researchers work on synthesizing derivatives that meet new toxicity or biodegradability standards. Pathways to greener feedstocks, like renewed focus on bio-based benzene or propylene, might one day trim the industry’s dependence on petrochemicals. Work also goes into studying blending with enzymes or biotechnology-based surfactants, carving out spots in green cleaning markets. Time after time, success often means collaboration across supply chains—feedstock producers, process chemists, and application engineers troubleshooting together, rather than fighting for their own pieces of the puzzle.
Toxicity Research
Most published animal studies paint sodium cumenesulfonate as having low acute toxicity. Testing on fish and algae highlights only moderate threats at high concentrations, far above those seen in typical wastewater. Chronic exposure data remains less abundant but enough to guide safe workplace practices. Actual experience in manufacturing has reinforced the need for containment, mainly to cut down on dust and mist inhalation, which can cause mild irritation. No signs point to meaningful bioaccumulation or persistence, but regulatory eyes stay focused on keeping cumulative loading of sulfonates in the environment to a minimum. Community engagement matters—people want reassurance that stormwater runoff won’t damage their local fishing hole or bird-watching marshland.
Future Prospects
The future of sodium cumenesulfonate will mirror trends in chemical manufacturing—a migration toward greener processes, safer handling, and better integration of lifecycle impact. As renewable feedstocks enter the mainstream, process chemists may find ways to lower the environmental footprint of cumene and the resulting sulfonates. Regulations continue shifting the market, especially in Europe and North America, where limits on aromatic hydrocarbon use keep tightening. Companies investing in smart process control are likely to cut emissions, waste, and energy use, all while delivering tighter product specs. Application markets like industrial cleaning, oil recovery, and construction chemicals seem stable, but growth depends on pivoting fast to regulatory change and new consumer priorities. For researchers, the challenge lies in answering tough questions about toxicity, fate in water bodies, and cross-over into consumer goods requiring lower thresholds of persistent organic pollutants. The technical road ahead is never smooth but stays open for those willing to balance reliability with innovation.
What Makes Sodium Cumenesulfonate Special?
Sodium cumenesulfonate crops up in a lot of places you might not expect. At the grocery store, in cleaning aisles, it sits tucked away as an ingredient in laundry detergents and all-purpose cleaners. It’s there for a good reason: helping liquids do their job better. This chemical doesn’t take the spotlight, but its role in getting dirt off your clothes or making kitchen counters spotless counts for plenty.
The Science Behind Its Use
This compound works by making it easier for water and oily, greasy substances to mix. It acts as a solubilizer, meaning it helps ingredients that usually don’t get along dissolve evenly. Picture a pot of soup: fat floating on top, water below. Sodium cumenesulfonate helps these layers combine, allowing detergents to break down grime more effectively. In my own kitchen, I’ve noticed the difference when using cleaners loaded with this stuff—no more cloudy residue or greasy streaks after wiping down the stove.
The cleaning industry isn’t the only place it shows up. Many personal care products—things like shampoos and bubble baths—use it for the same reason. Skin feels cleaner, and you don’t get that filmy feeling some soaps leave behind. It also acts as a thickener, giving shampoos a satisfying, rich texture. Considering how often people touch these products, using ingredients that actually improve the experience matters for both companies and consumers.
Environmental and Health Factors
Every year, millions of gallons of cleaning fluids and shampoos flow down the drain. Researchers have looked closely at what happens to ingredients like sodium cumenesulfonate once they reach water treatment plants. Toxicity studies found that it breaks down pretty well in the environment—microbes in sewage systems chew it up rather quickly. For most folks, the health risks tied to this ingredient stay pretty low, with skin irritation being rare and only in concentrated form. Still, no chemical is perfect, and being mindful of what ends up in streams and rivers never hurts.
It surprised me to find out that manufacturers keep tabs on how much they use. The Responsible Care program, for example, pushes companies to keep ingredients like this from building up in the water supply. While everyday people might not think twice about these behind-the-scenes checks, knowing there’s a system for oversight offers peace of mind the next time you do a load of laundry.
Moving Toward Greener Formulas
With pressure to go green, the cleaning industry is trying to swap out older chemicals for milder, plant-based alternatives where possible. The market for environmentally friendly cleaners jumped 30% in the past decade, because consumers started reading labels and demanding safer ingredients. Sodium cumenesulfonate often makes the cut since it fits the bill for safety and performance. It lets companies reduce harsher solvents while keeping products effective. From my perspective, making smart substitutions like this strikes a good balance: clean surfaces, fewer worries about what ends up in waterways. That’s a shift everyone can benefit from, whether you’re scrubbing floors or washing your hair.
Insight into Everyday Chemicals
Walking down any store aisle and reading ingredient labels, it’s easy to feel overwhelmed by names like sodium cumenesulfonate. Many people might wonder what this compound does and if it’s something they should worry about in their skin care products. The truth is, sodium cumenesulfonate shows up in household items for a reason. It plays a role in mixing oil and water, helping products rinse out easily, and sometimes boosts how well other ingredients work. You’ll spot it in shampoos, soaps, dish detergents, and even cleaners.
Digging Into the Research
Questions about safety come up for good reason. People want to avoid irritation, allergic reactions, or any long-term issues. The Cosmetic Ingredient Review (CIR) Panel, a group known for evaluating the safety of ingredients, has reviewed sodium cumenesulfonate. They consider both its chemical structure and real-world use. According to their findings, this ingredient does not react with skin in the way some harsher cleansing agents do. Most reports suggest low risk of irritation, even for people with sensitive skin.
Backing up these reviews, toxicology reports point to several important findings: sodium cumenesulfonate shows little to no absorption through healthy skin, and tests show it doesn’t trigger genetic changes or promote cell damage in typical usage amounts. Product manufacturers rarely use it in high concentrations, usually keeping it at under 5%. That keeps exposure limited, lowering the chance of irritation or allergic response.
Looking for Red Flags
While sodium cumenesulfonate scores well in skin safety profiles, not everyone’s skin reacts the same way. People with eczema, psoriasis, or allergies need to keep an eye out for any new symptoms. Patch testing tells a good story here—try a small amount, wait for a day or two, then decide if the rest of the product works for you.
In my own experience working with people who have chronic skin conditions, anxiety stems less from a single ingredient and more from piling on multiple cleansers or treatments that strip the barrier. Overcleansing or using hot water with any surfactant can cause problems. Sodium cumenesulfonate doesn’t land high on my worry list, but I always suggest looking at overall routines rather than fixating on unfamiliar names.
The Role of Transparency
Brands today face growing pressure to talk straight about their formulas. Detailed ingredient lists and plain-language explanations matter. Sharing how much of something goes into a product gives peace of mind. Several skin care companies include not just the name but also describe the role and percentage, plus any related research. These steps go a long way in building trust, especially with people who have already dealt with stinging or rashes.
Sensible Steps and Sound Choices
For anyone hoping to avoid irritation or allergies, simple choices go far. Stick to products from reputable brands that publish their safety data, and avoid mixing too many “intensive” products at once. Think about skipping any new item if your skin already feels raw. If redness or bumps turn up, stop use and talk to a dermatologist about what might have gone wrong.
Overall, everything points to sodium cumenesulfonate being a low-risk option on skin in typical products. Like with any ingredient, watching how your skin responds matters most.
Breaking Down Its Physical Character
Open a sealed drum of sodium cumenesulfonate, and you’ll probably notice a white or off-white powder or granule. It usually doesn’t carry much of a smell, much less something irritating. If you were to toss it in a glass of water at room temperature, you’d see it dissolve quickly. Its solubility makes life easier for anyone mixing solutions—no endless stirring or waiting for clouds to clear. From my time in the lab, the difference that makes can’t be overstated, especially when you’re on a schedule or dealing with big batches.
Touch it, and it feels a bit gritty, but not oily. It doesn’t cake unless badly stored. Some chemicals always seem to find a way to clump up or draw water from the air, leading to storage headaches. Sodium cumenesulfonate avoids that pitfall, which matters in warehouses where humidity isn’t always ideal.
Chemical Properties and What They Mean
At the molecular level, sodium cumenesulfonate contains a benzene ring, a sulfonate group, and a sodium ion. The chemical formula for those keeping track is C9H11NaO3S. That aromatic ring gives the molecule stability and lets it play a part in reactions requiring an aromatic backbone. The sulfonate group, carrying a negative charge, grabs onto water molecules and works well as a hydrotrope. Basically, it helps other ingredients dissolve, especially oily or stubborn chunks that fight water.
It usually hangs tight at a near-neutral pH when fully dissolved—think pH 7 to 9. That means it mixes safely with many other cleaning chemicals without setting off unwanted side reactions. During my days troubleshooting formulation glitches, that stability saved time and reduced wasted raw materials.
On the heat front, sodium cumenesulfonate stands up to higher temperatures without breaking down fast. Many cleaners or degreasers hit hot surfaces, so this property keeps performance steady. Around strong acids or oxidizers, you do get some risk—beyond what you’d see with plain table salt but not wildly more dangerous. Common sense handling, gloves, and goggles keep you safe.
Real-World Uses and Everyday Value
Most folks come across sodium cumenesulfonate without realizing—maybe in a bottle of hard surface cleaner, detergent booster, or industrial spray. Its strength lies in keeping stubborn dirt in the mix, so it helps detergents actually do their job. Water alone rarely cuts through heavy grease; adding this hydrotrope makes a clear difference. Some manufacturers even slip it into paints or coatings to help additives disperse without creating lumps or color streaking.
I’ve seen sodium cumenesulfonate shine in places plagued by hard water. It doesn’t soften the water, but it lets cleaners do their job better, reducing visible streaks and residue. As more factories look to limit phosphate-based boosters for environmental reasons, sodium cumenesulfonate stands in as a friendlier option.
Potential Hiccups and Smarter Solutions
Not everything sparkles with sodium cumenesulfonate. Large spills can make surfaces slippery, and overuse in wastewater can challenge municipal treatment. Scientists and industry pros continue to study its path in the environment, aiming to close those gaps. Some labs are also checking how tweaks to the molecule might make it break down faster after use, or how blends with natural hydrotropes could boost performance without side effects.
Finding better storage solutions, updating labels, and offering more worker training could cut down unwanted exposure or handling mistakes. Regular review of newer, safer alternatives pushes everyone forward without letting down performance or safety.
Wrapping Up with the Essentials
Sodium cumenesulfonate brings practical advantages thanks to how it behaves physically and chemically. Its quick dissolving, resistance to caking, and hydrotropic punch help across industries, tackling real, day-to-day cleaning jobs. Thoughtful development and continued safety conversations keep it as a reliable choice while supporting the move to safer, greener chemistries.
What Happens to Sodium Cumenesulfonate After We Use It?
Sodium cumenesulfonate slips into a wide range of cleaning products—everything from laundry detergents to industrial degreasers. I learned about it by reading the fine print on a bottle of floor cleaner after my daughter spilled grape juice on the carpet. I started to wonder what happens to all these chemicals once they wash down the drain. Some folks don’t give it much thought, but the answer touches how we safeguard water and soil in a world that produces an astonishing amount of wastewater.
Is It Biodegradable?
Straight to the big question: sodium cumenesulfonate does break down in the environment, but at a moderate pace. Data from biodegradation studies report about 60% breakdown within 28 days in standard OECD tests. This puts it in the “readily biodegradable” camp by some official classifications, but the real world isn’t always as neat as a lab dish. Temperature, sunlight, microorganisms, and dilution all factor into the environmental fate. Sometimes, what disappears rapidly in a lab stays stubborn in a cold, nutrient-starved riverbed, especially during winter or drought.
Why Biodegradability Matters for Everyday Life
From what I’ve seen growing up near a river that had its share of pollution scares, chemicals that linger cause trouble. If compounds like sodium cumenesulfonate didn’t biodegrade, they might collect in river sediment or groundwater, eventually causing harm to plants, fish, and those of us relying on that water. The risk goes up as more households and industries pour detergents down the drain. Even “biodegradable” substances can build up faster than they break down if people keep using them by the ton, which happens, especially in big cities or in agriculture. Everyone who lives downstream could feel those impacts, whether they taste a difference in their drinking water or see fish populations change.
The Bigger Picture: Regulations and Safer Chemistry
Agencies like the European Chemicals Agency and the U.S. EPA pay close attention to the breakdown profiles of chemicals in commerce. Their regulations mostly steer companies towards safer options, but the public and manufacturers both push for products that deliver cleaning power without extra environmental baggage. In Europe, surfactants used in detergents have stricter rules—requiring primary biodegradation above 80%. Sodium cumenesulfonate falls just short in some test setups, which leads some formulators to blend it with even safer agents or limit long-term use. There’s market interest in eco-labels and certifications, which often require proof of low toxicity and fast breakdown.
Looking Toward Solutions
I hear from chemists and environmental engineers working on next-gen surfactants that start with plant-based ingredients, which the environment breaks down more quickly and completely. Some companies research tweaking the structure of additives to help microbes gobble them up faster. On the user end, people can check labels or search for brands that publish environmental impact data. Municipalities invest more in wastewater treatment technology these days, with a nod to biological processing that filters out more surfactants before the water cycles back into rivers. Those actions add up, whether it’s a parent reading ingredient lists, a scientist working in the lab, or a city building a new filtration system. Even a small move toward mindful choices helps tip the scales for cleaner water, healthier ecosystems, and a bit less worry after mopping up grape juice spills.
Why Attention to Storage Pays Off
My first run-in with chemical storage issues came early, in a cluttered supply closet mixing all sorts of stuff. Someone stored a sensitive compound by an open window; the sunlight and moisture visiting the jars almost every day. That’s how I learned not to shrug off storage guidelines. Sodium cumenesulfonate might look like a stable powder or granule, but keeping it right means fewer safety headaches and more reliable performance over time.
Keeps Its Cool and Stays Dry
Good chemistry starts with dryness. Humidity creeps in and can clump just about anything, sodium cumenesulfonate included. Once clumped, you get trouble dosing it properly or blending it into solutions without a fight. It never hurts to stash it somewhere cool, either. Room temperature usually works, but think closer to 20-25°C, far from a warm radiator or sunny shelf.
You won’t see explosions or fires from sodium cumenesulfonate lying around a few extra degrees warm, but rising temps don’t do it any favors. Extended heat exposure can degrade lots of organic salts, sometimes before you even realize there's a problem. Anyone hoping for long shelf life should skip attics, garages, or any spot that swings hot and cold throughout the year.
Avoiding Contamination and Chemical Surprises
Shared storage shelves make things more complicated. Stacking chemicals side by side often works fine, but acids, strong oxidizers, and other reactive substances can spell trouble. Even with sodium cumenesulfonate’s fairly mild profile, cross-contamination can lead to foul odors or discoloration in the product, which shows poor stewardship more than anything else.
Locking the original packaging after each use goes a long way. I’ve seen folks stretch the neck of a bag, tie it off haphazardly, and come back to a lumpy, sticky mess. Resealing makes a difference. For business-scale amounts, sealed drums with tight lids keep things proper year after year.
Safety Wins Friends, Legal Compliance Seals the Deal
Even if sodium cumenesulfonate doesn’t sit on the most hazardous lists, the rules don’t bend for convenience. Occupational Health and Safety standards everywhere spell out dry, cool, and ventilated environments for storing organic salts. It doesn’t just protect the chemical—it protects workers and minimizes complaints after audits. Safety data sheets echo the same advice, giving anyone in the loop a common playbook.
Strict lab managers always check for properly labeled shelves, remember to keep incompatible materials apart, and encourage quick cleanup of any spill. In big operations, regular inspections catch minor leaks in containers before they turn into bigger issues.
Practical Solutions for Secure Storage
Taking extra care just takes a little planning. Store sodium cumenesulfonate in tightly sealed containers, keep it on a shelf out of direct sunlight, and stick to moderate temperatures. Use dedicated spaces away from acids and bases; keep everything dry to avoid ruined powder and sticky messes. Read the label, trust the safety data sheet, and ignore shortcuts that trade safety for convenience.
This kind of attention means less wasted product, consistent results, and fewer downtime interruptions. That’s more time focused on the work that matters, instead of cleaning up avoidable messes.