(S)-(+)-3-Hydroxytetrahydrofuran: A Deep Dive into Its Journey and Impact
Historical Development
Many chemical discoveries that seem ordinary today started with a puzzle that challenged both curiosity and skill. In the early days of stereochemistry, researchers looked for ways to isolate single enantiomers from racemic mixtures. The search for optically pure compounds shaped pharmaceutical chemistry, and (S)-(+)-3-Hydroxytetrahydrofuran emerged as a small but key molecule with unique chiral properties. Early reports from the late 1900s highlighted the need for compounds like this in asymmetric synthesis, pushing chemists to refine resolution methods and asymmetric catalytic approaches. By the mid-2000s, a wave of interest in sustainable, enantioselective routes brought new attention and practical, scalable syntheses.
Product Overview
(S)-(+)-3-Hydroxytetrahydrofuran shows up in research labs and production lines dedicated to pharmaceuticals, natural product synthesis, and advanced materials. Its chiral hydroxyl group is prized for introducing asymmetry in drug intermediates and target molecules. Researchers value this molecule for its role in stepwise organic transformations, where control over stereochemistry can determine the difference between a life-saving drug and a failed candidate. Labs in both academic and industrial settings trust its consistency, using certified suppliers who document not just purity but also traceability and reproducibility, echoing regulatory and research requirements.
Physical & Chemical Properties
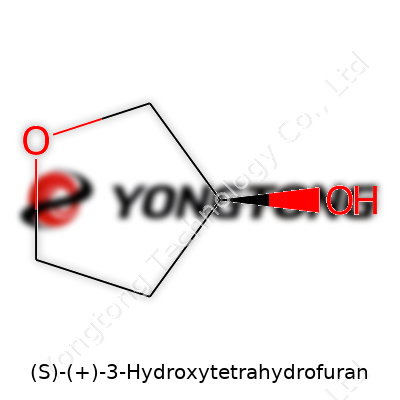
This compound arrives as a colorless to pale yellow liquid. It tends to have a faint, ether-like odor. With a molecular formula of C4H8O2, the structure forms a five-membered oxygen-containing ring with a single chiral center at the three position. Boiling point generally sits between 162 °C and 170 °C under atmospheric pressure, reflecting the balance of hydrogen bonding and ring strain. Water solubility remains high enough for most laboratory purposes. Chemical stability holds up under neutral and mildly basic conditions, though strong acids or bases can cleave or ring-open the molecule. Rotational measurements using polarimetry confirm the chirality; only the S-enantiomer shows the right positive optical rotation value, a critical check before downstream use.
Technical Specifications & Labeling
Quality assurance for (S)-(+)-3-Hydroxytetrahydrofuran involves tight controls on enantiomeric excess (often over 98% ee), residual solvent levels, and trace impurities. Standard labeling reports batch numbers, lot-specific analytical data, and safety hazards including GHS pictograms and precautionary codes (e.g., flammable, irritant). Manufacturers supply Certificates of Analysis (COA) listing NMR spectra, IR reference peaks, and chiral HPLC results. Labels also specify storage recommendations, typically cool and dry, shielded from sunlight, and away from incompatible substances like oxidizers. Some suppliers highlight compliance with REACH and other international chemical safety legislation, as the push for regulatory alignment grows.
Preparation Method
Chiral building blocks like this start from simple biomass-derived feedstocks or by resolution of racemic mixtures. Catalytic asymmetric hydrogenation, enzymatic kinetic resolution, and biocatalytic reduction rank high among preparative methods. For example, starting with racemic 3-hydroxytetrahydrofuran, resolution through chiral acid addition generates diastereomeric salts, which are separated and hydrolyzed. More scalable, green chemistry approaches use engineered enzymes or chiral transition metal complexes, slashing waste and sidestepping dangerous reagents. Experienced process chemists design steps to minimize cost and maximize enantiomeric purity while monitoring yield, sometimes reprocessing mother liquors to squeeze out extra product.
Chemical Reactions & Modifications
(S)-(+)-3-Hydroxytetrahydrofuran demonstrates both reactivity and selectivity. The primary alcohol group allows for easy oxidation to aldehydes or carboxylic acids, or protection as a silyl or benzyl ether. Ring-opening reactions with nucleophiles produce linear diols, while activation with tosyl chloride provides a platform for further substitution. Under acidic or basic catalysis, the tetrahydrofuran ring can expand or rearrange, sometimes yielding pyran derivatives. Using strong oxidizing agents pushes the molecule toward fragments that feed into synthetic schemes. In my own graduate work, I leaned on this molecule as a chiral auxiliary in diastereoselective reactions, finding that its balance of chemical stability and predictable reactivity made it a reliable workhorse in multistep organic synthesis.
Synonyms & Product Names
Chemists bump into a wide range of labels, from systematic to plain trade names: (S)-Tetrahydrofuran-3-ol, (S)-3-THF-ol, (S)-(+)-3-HTHF, 3-hydroxy-oxolane (S), and even left-handed tetrahydrofuran-3-ol. Common chemical catalogs will often display CAS numbers and reference both enantiomeric forms, demanding close attention to avoid costly errors. In commercial contexts, the “S” prefix and explicit sign of optical rotation act as shorthand that saves time and avoids miscommunication.
Safety & Operational Standards
Working with this material in the lab means reviewing both flammability and possible skin or eye irritation hazards. Inhalation of vapors requires solid ventilation or fume hoods. Protective gloves, lab coats, and goggles cut down on splash risks—my own time in the lab left no doubt that a little caution keeps the day going smoothly. For large-scale synthesis, equipment grounds and inert atmospheres reduce fire risk. Spillage protocols line up with solvent-category containment: absorbents, engineering controls, then secure containerization for waste disposal. Training on practical GHS and local chemical hygiene plans stays essential for anyone handling this or similar fine chemicals.
Application Area
Pharmaceutical research draws heavily on (S)-(+)-3-Hydroxytetrahydrofuran for manufacturing active pharmaceutical ingredients that demand specific chirality. Its presence in antiviral and cholesterol-lowering drug syntheses highlights a broader shift: companies realize the push for pure enantiomers makes both medical and business sense, as regulatory bodies tighten standards. Beyond pharma, polymer chemists incorporate this building block into specialty materials where stereochemistry influences final properties, such as optical activity or bioresorbability. Flavors and fragrance formulations use it for intermediate steps that build complex aroma molecules; here, purity and traceability maintain both performance and safety in consumer goods.
Research & Development
The scientific community keeps busy with ways to streamline production, improve yields, and lower environmental impact. Teams look to biocatalytic methods, cleverly engineered microbial strains, and continuous flow reactors for answers. In my own network, collaborations between industry and academia push research from milligram to kilogram scales while managing energy costs and waste. Patent landscapes show intense activity around novel catalysts and process intensification, while journal articles keep highlighting both incremental and breakthrough improvements. Funding agencies prioritize greener approaches, supporting pilot programs that could scale well past the lab bench.
Toxicity Research
No one can ignore the need for rigorous toxicity screening, especially with chiral chemicals entering biomedicine. Standard animal and cellular studies show low acute toxicity, but careful chronic studies track possible metabolic byproducts and susceptibility in sensitive populations. Regulatory filings in the EU and US require detailed toxicokinetic profiles, so development programs invest in comprehensive data packs. Lab personnel run exposure drills and maintain up-to-date MSDS documentation. A few structural analogs show mild irritancy, but no genotoxic or carcinogenic risks have flagged up in standard panels so far. Still, research never assumes complete safety, pushing for ongoing updates as new analytical tools arrive.
Future Prospects
The forecast for (S)-(+)-3-Hydroxytetrahydrofuran points to expanded use, driven by demand for chiral drugs and tight supply chains in fine chemicals. Synthetic organic chemists keep probing for faster, cheaper, and greener ways to access this chiral synthon. Major producers look for business opportunities in contract manufacturing, where process reliability meets strict regulatory demands. Biotechnologists develop sustainable enzymatic pathways that not only improve yields but also sidestep hazardous waste. Integration with digital process control and AI-driven reaction optimization hints at smarter, more resilient chemical manufacturing. With 3D cell culture and novel drug platforms on the rise, this compound stands poised to play a supporting role in both old and new chemistries shaping tomorrow’s healthcare, consumer products, and high-value polymers.
Behind the Lab Door: The Story of a Versatile Compound
Some chemicals draw a lot of attention only behind locked lab doors. (S)-(+)-3-Hydroxytetrahydrofuran, or simply 3-HTHF, belongs to this family. Spend enough time with chemists, and you’ll notice a special regard for molecules like this one. It comes from the world of chiral chemistry, which stirs excitement for anyone shaping or improving drugs, both generic and new. Many life-saving medications depend on these odd-shaped molecules to work as intended in the human body.
In my time talking to researchers, no one claims this compound as a miracle substance. Instead, they talk about quiet reliability and precision. What (S)-(+)-3-Hydroxytetrahydrofuran brings to the table is its role as a building block. Chemists reach for it when they want to create more complex molecules with a precise “handedness” – that subtle trait that occasionally turns a safe medicine into a harmful one if not just right. Our own body prefers specific shapes at the molecular level. So, chiral purity means fewer side effects, greater effectiveness, and safer drugs.
Pharmaceutical Use: A Building Block, Not a Buzzword
Pharmaceutical researchers find value in 3-HTHF for one main reason: synthesis of intermediate compounds. Think of it as a Lego brick, used to snap together custom shapes for antivirals, antibiotics, or nerve agents. For example, this molecule helps create nucleoside analogues, critical in the fight against viruses like HIV or hepatitis. Some teams working on cancer therapeutics enjoy the convenience and reliability it brings to their process, keeping batch consistency high and reducing time spent on tricky purification.
From a first-hand perspective, chemists like having ready access to such compounds. The fewer steps between raw material and finished drug, the better for keeping costs in check and avoiding chemical waste. This directly impacts drug development speed and how fast patients get relief. The regulatory world recognizes the value, too. Purity and reliable sourcing have their place in every filing submitted to agencies like the FDA or EMA.
Beyond Medicine: Niche Uses with Real-World Impact
Not every story about 3-HTHF involves a pill or a shot. Inside specialty labs, it’s used in the synthesis of flavors and scents, helping give perfumes or food supplements their edge. Those applications might seem minor but play key roles in the industry, supporting product safety and creative effort. As a solvent, 3-HTHF doesn’t show up as much as classics like acetone, but it finds favor in settings where chiral purity must stay untouched.
Dealing with Safety and Supply Issues
Handling chiral compounds gently remains a challenge. Many researchers have faced shortages or price hikes, especially for enantiopure chemicals. Reliable supply chains matter here more than in some other areas: even minor errors can ruin batches and delay entire trials. Labs need clear documentation, batch history, and certifications from trusted suppliers. Digital transparency and continued investment in synthesizing greener, more scalable versions of the molecule offer a path forward.
People sometimes underestimate the ripple effect these foundational chemicals have on science and industry. My own discussions with colleagues remind me how even small tweaks to cost or sourcing can halt entire research programs or keep breakthrough drugs on the shelf. Investing in robust supply networks and green chemistry reduces both risk and environmental impact, ensuring more scientists get to build what they envision.
The Real Value of Purity
Every chemist I know looks past the glossy summary datasheet when it comes to chiral building blocks like (S)-(+)-3-Hydroxytetrahydrofuran. Here, purity is not just a technical detail—it's the guardrail between a successful reaction and a costly rerun. In practical terms, most reputable suppliers post purity values upwards of 98%. I have come across listings promising even higher grades, hitting the 99% mark. Still, numbers on a label tell only part of the story. Shelf life, handling during shipping, and even small differences in batch processing can all nudge that figure up or down without warning. Lab experience has taught me that verification beats blind trust every time.
Why Purity Shapes Experiments
Every step away from true purity means an extra variable in the flask. Contaminants, even in tenths of a percent range, can twist the outcome, sidetrack chiral resolution, or give false readings in bioassays. In my own work, especially on asymmetric syntheses or scale-up studies, using material even slightly below spec has ended up tanking yields or muddying enantiomeric ratios. This is not just academic. It translates directly to wasted time, lost funding, and extra runs in the rotavap hoping to salvage something usable.
How to Judge Supplier Claims
I never rely on a single Certificate of Analysis (CoA). Cross-checking with independent NMR reports or even HPLC traces is a habit born from seeing surprises turn up more than once. Some suppliers expect no one to look past the printed number. I have seen batches labeled at 98% that, under closer inspection, carried visible side-product fractions. For researchers scaling up, those impurities magnify with every gram. A purity dip to even 95% ramps up the load of byproduct and makes downstream purification tougher. That eats into overall productivity, not to mention the headache of tracking down why a particular synthesis failed when the reagents “should” have been up to spec.
Quality Control as a Ongoing Commitment
In the fastest-moving labs, folks often slip into trusting the most familiar vendor out of convenience or habit. It pays to keep records of batch-to-batch results and, if possible, request analytical data for each shipment, not just the first one. If questions ever arise about purity, sending the sample for third-party analysis quickly becomes the difference between a successful series of reactions and weeks lost on troubleshooting. Sharing these results across a team also lifts everyone’s understanding—nobody likes repeating the same mistakes their colleague made last quarter.
Better Practices Going Forward
Chasing higher purity pays off in less rework and more confidence with every step of a synthetic route. For sensitive work, it makes sense to negotiate extra purity checks—even GC or MS analysis—before money changes hands. Putting effort into tighter QA processes lifts the whole research group and earns trust downstream in collaborations or product development. Personality matters here: direct, open conversations with account reps about real-world outcomes often lead to better supply relationships and fewer surprises. The stakes are too high to let assumptions about purity slide.
Why Proper Storage Really Matters
Every chemist who’s spent time with chiral intermediates has felt that little prickle of anxiety when the bottle gets left out too long or the storeroom gets stuffy. (S)-(+)-3-Hydroxytetrahydrofuran is one of those quirks of the lab: useful, not wildly unstable, but still asking for a bit of respect. Leaving its storage up to chance risks losing purity or, worse, ruining a whole string of downstream reactions.
Finding the Right Spot in the Lab
This solvent-like liquid brings a faint ether aroma, pointing to its volatility and sensitivity—this has always flagged up red lights for me after too many nights cleaning up rings and ethers gone off. Sticking the bottle in a cool, dry place keeps its properties true to form. I always recommend room temperature or just slightly cooler, away from sunlight. If sunlight strikes the bottle, even clear glass, there’s a chance oxidation or slow decomposition will set in.
Oxygen and moisture aren’t a friend here. Left uncapped for long, (S)-(+)-3-Hydroxytetrahydrofuran can absorb water from the air. This dilutes the concentration and introduces risks in moisture-sensitive syntheses. After years working alongside pharma chemists, the sound advice that stuck with me: tightly close the cap as soon as you pour, and use an inert gas like nitrogen to flush the headspace if the bottle stays open for longer periods.
Hints for Longer-Term Storage
In my own teaching days, I watched students overlook these basics and always regretted letting things slide. If the material isn’t for immediate use, refrigeration around 2–8°C keeps degradation in check. Freezing brings risks of cracked containers and condensation, so I avoid the freezer unless the supplier says the material can handle deep cold.
Containers do most of the heavy lifting: high-quality amber glass protects against UV. Low-density polyethylene, while cheap, can sometimes leach plasticizers—stick to glass if you want consistent results or if there’s any chance your work will reach a clinical or regulatory checkpoint. Always label every bottle with the date received, date opened, and who opened it. This helps you track any changes and spot patterns if things go off.
Possible Hazards and Smart Solutions
A strong odor often signals evaporation, and, like with many tetrahydrofuran derivatives, flammability can’t be ignored. Storing far away from open flames, hot plates, or direct heat sources reduces risk. I remember one aging storeroom where a hot steam pipe nearly ended in disaster after a few misplaced ether bottles spent summer afternoons sweating.
If I had to suggest just one improvement for safer storage, it’s training. A five-minute rundown before any project begins, and reminders at every safety talk, can spare hours of lost work and wasted reagents. Posting clear guidelines near storage areas helps everyone remember: dry, cool, tightly sealed, and away from light.
For research teams handling high-purity chiral intermediates, scheduling regular audits of all storage areas catches problems early. If a bottle’s label smudges or a cap’s loose, it gets resolved before the team’s project hits a snag.
Building Good Lab Habits Pays Off
My own career has been shaped by small, consistent steps, rather than dramatic actions. (S)-(+)-3-Hydroxytetrahydrofuran isn’t notorious for instability, but careful storage protects results, budgets, and reputations. By giving storage the same respect as synthesis, any lab can keep their science moving forward—with less waste and fewer risks.
Not Your Everyday Chemical
Ask most chemists about (S)-(+)-3-Hydroxytetrahydrofuran and they’ll probably raise an eyebrow. It isn’t a staple like acetone or ethanol, but its role in chiral synthesis and drug research lets it punch above its weight. I remember first seeing it in an obscure journal during my graduate days—its unique configuration made it shine in some challenging enantioselective syntheses. Fast forward, and the interest keeps growing, especially for those fine-tuning specialty molecules or trying to shave months off new pharma development.
Supply Chain Realities
If a research team needs a gram or two, specialty catalogues like Sigma-Aldrich or TCI often deliver. Bigger needs—say, several kilos or even barrels for advanced manufacturing—send folks into deep supplier hunts. Reliability matters, since chiral intermediates rarely let you switch gears at short notice. Until recently, complaints circled about wait times or suppliers forced to synthesize on-demand, quoting months before shipping.
Who's Got It in Bulk?
A few big players—think specialty arms of Merck or Chinese custom factories—offer bulk (S)-(+)-3-Hydroxytetrahydrofuran, though not without a catch. Quality standards jump around from supplier to supplier. Some batches nail the optical purity; others need additional work. Pricing can swing wildly too, depending on demand or production run size.
The truth: bulk volumes exist, but not with the consistency of commodity chemicals. Drug makers and advanced materials labs working in the kilo-to-ton range still vet vendors like crazy. Certifications, purity profiles, and supply contracts matter more than ever. If a clinical synthesis depends on this chiral building block, teams often ask for custom runs with extra quality checks.
Why This Matters Beyond the Lab Bench
Bulk availability of obscure building blocks like (S)-(+)-3-Hydroxytetrahydrofuran shapes how fast new medicines reach patients. Delays in procurement, repeated purity issues, or failed shipments can stall entire programs. I once worked with a startup that nearly missed a funding milestone because the bulk supplier underestimated delays in chiral intermediate delivery. In the pharmaceutical arms race, slowdowns like this cost real money—sometimes the company.
On an industry scale, higher capacity distribution helps strip away those barriers. Companies able to scale up or cut lead times build resilience into the whole sector. For custom synthesis firms, investments in process development—not just sales claims—pay the biggest dividends. A transparent supply chain lets buyers see exactly what’s on offer, and organizations like the European Medicines Agency often insist on traceability to keep standards high.
Room for Improvement
One clear solution involves fostering partnerships between buyers and chemical makers early, so neither side risks surprises late in the game. Companies who keep teams in touch, share technical challenges, and invest in longer-term deals secure their own timelines and save resources down the line. Digitalization of supply listings can go further. Direct, verified access to real-time inventories cuts out weeks of email ping-pong, and helps research teams forecast their needs more precisely.
Quality doesn’t just mean ticking regulatory boxes. It’s about giving scientists room to do their best work, without last-minute uncertainty over batch rejections or shipment timelines. As global scientific communities push up demand for niche chiral intermediates like (S)-(+)-3-Hydroxytetrahydrofuran, the firms who offer clarity, transparency, and genuine expertise in scale-up will set themselves apart.
Why a CAS Number Matters in Real Life
There's a world behind every small bottle or chemical container we see in a lab. Instead of calling a compound by a long technical name, scientists and workers rely on a short string of numbers called a CAS number. The number carries a lot of weight. Whether tracking shelf inventory or buying from a chemical supplier, the CAS number removes guesswork. For (S)-(+)-3-Hydroxytetrahydrofuran, the unique CAS number is 86087-23-2. Anyone searching databases, safety sheets, or regulatory info will get the right hit if they plug in this number.
Tracking Safety and Quality With Confidence
Every chemical in circulation must have solid traceability. I’ve worked in settings where even a slight mismatch in chemical identity meant wasted time and tight budgets. Regulatory bodies like the FDA or EPA keep eyes on chemical data with these numbers. When researchers want to read up on toxicology or hazards, the CAS number points them to the right records, and safety teams stay ahead on rules and safe handling guidelines.
What Makes (S)-(+)-3-Hydroxytetrahydrofuran Stand Out
Chemists choose (S)-(+)-3-Hydroxytetrahydrofuran for a reason. Its ring structure and chiral center open up a range of possibilities for new drug compounds or specialty materials. This molecule helps in building blocks for pharmaceuticals or advanced materials for electronics. The world doesn’t see these chemicals directly, yet their fingerprints show up in devices and medicines people rely on daily.
Even though the chemical name sounds like a mouthful, the CAS number simplifies things. Instead of stumbling through pronunciation or spelling errors, folks in purchasing or research pop 86087-23-2 into their platforms — zero confusion, no time lost. It’s a practical solution built for real-world labs and businesses.
Challenges in Handling and Purchasing
Not every supplier labels bottles the same way, and mistakes can happen. I’ve seen cases where similar names led to dangerous mix-ups. Subtleties in naming, like (S) versus (R) forms, aren’t just academic points. In pharmaceuticals, the wrong form can change how a drug works or trigger side effects. The CAS system brings clarity: one number, one structure, matching regulatory files, supplier records, and safety protocols.
Sourcing chemicals without the CAS number risks more than financial loss. There’s a hazard lurking if someone handles the wrong stereoisomer, or uses a substitute without realizing the change in properties. In collaborative projects, I’ve seen teams at different sites avoid costly errors simply by confirming CAS numbers before shipping or using a batch.
Keeping Science and Industry Accountable
Bigger themes surround these small identifiers. Transparency and traceability keep everyone honest. Manufacturers, health authorities, and researchers depend on an unbroken chain from product origin to finished result. If a safety recall pops up, the CAS number leads the way in tracking down affected lots.
Care in documenting and sharing accurate numbers like 86087-23-2 for (S)-(+)-3-Hydroxytetrahydrofuran helps maintain trust, protects public health, and keeps innovation steady. Science wins when these standards get followed—no shortcuts, just clear information flowing from discovery to production lines to safety audits.