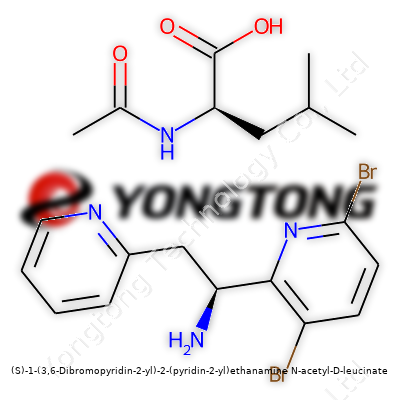
(S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate: An In-Depth Commentary
Historical Context and Scientific Roots
Nothing about (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate just happened overnight. This compound ties back to the decades-old pursuit to synthesize selective agents for neurological research and potential therapeutic applications. Chemists started mixing pyridine derivatives with amino acid conjugates years ago, not just out of curiosity but out of necessity. Interest really took off after early studies pointed to the versatility of brominated heterocycles in medicinal chemistry. Tweaking the pyridine ring in the lab opened doors in receptor- and enzyme-targeted drug discovery, but these advances didn’t come easily. Whether you’re flipping through mid-1990s neuroscience journals or specialist patents from Japanese labs, you see the pattern: iterative design, practical setbacks, some genuine scientific luck. The backdrop here isn’t just about innovation—it’s grit, resourcefulness, and an evolving sense of what these molecular hybrids can deliver.
Product Insights
You’re dealing with a multi-component molecular structure. The ethanamine core sits tightly bound up with two pyridine rings—one with dibromo substituents at the 3 and 6 positions, the other left unadorned. On top of that, N-acetyl-D-leucinate comes into play, pulling from chiral amino acid chemistry to influence solubility, target selectivity, and bioactivity. No chemist would look at this thing and think “stock reagent”—it’s deliberately built for complexity and specificity. Commercial sources of the compound, when you can find them, almost always get routed to research organizations, early pipeline pharma projects, and sometimes boutique chemical suppliers working with universities. It’s not often sitting shelf-side at the big box chemical warehouses, partly due to specialized demand, partly because of the extra handling measures required for halogenated pyridine derivatives.
Physical and Chemical Foundations
Physically, this compound has a solid-state crystalline form under ambient conditions, and moisture tolerance leans on the robust amine and amide groups. The molecular weight tips over 490 g/mol, mostly because of those two hefty bromine atoms. Bromination usually impacts melting point and volatility, and here that means the compound offers solid handling at standard temperature, but you don’t want to leave it uncapped for long. The structure’s aromatic character and the presence of multiple nitrogen donors bring in strong binding affinities for metal ions and biological targets. Chemically, you find it’s stable under refrigerated conditions and in the dark, but light or aggressive acids can coax out some degradation. Solubility sits in the moderate organic range, with DMSO and DMF working best; water doesn’t get much solubilization beyond trace levels thanks to the hydrophobic aromatic core.
Technical Characteristics and Labeling Realities
Labels for materials like this don’t mince words. You’ll spot full IUPAC names, CAS numbers, batch records, and storage instructions running right across the packaging. Purity promised by suppliers often tracks above 98%, but real-world checking with NMR or mass spectrometry still matters, since minor contamination carries risk for sensitive assays. Well-run labs know to check for the presence of isomeric side products. Documentation tends to include MSDS data—a must for compliance regulations—highlighting proper handling gear, ventilation needs, and first aid measures. Any facility storing the compound follows up-to-date GHS labeling, showing off the compound’s risk profile as a halogenated aromatic, not just a generic organic molecule.
Preparation Pathways
Making (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate starts with multistep synthesis. Most chemists begin by brominating pyridin-2-yl precursors using controlled addition of Br2 in anhydrous conditions, then moving to Suzuki or Stille coupling to build the biaryl platform. The ethanamine chain gets introduced using a reductive amination strategy, grabbing hold of the S enantiomer for chirality. Attaching the N-acetyl-D-leucinate moiety relies on peptide coupling reagents—carbodiimides or uronium-based activators do the trick. Chemists then purify with silica gel chromatography and crystallize from ether-hexane or acetonitrile. Yields often vary because every subtle change—temperature swings, solvent choice, work-up technique—affects racemization and byproduct formation. Best practice involves ongoing TLC or HPLC monitoring at every stage, something I’ve learned makes or breaks batch reproducibility.
Chemical Modifications and Typical Reactions
Anyone working with this molecule quickly finds that, despite its stability, the compound’s reactivity opens doors for downstream modifications. Nucleophilic aromatic substitution on the brominated pyridine can let in a range of functional groups, including alkoxy and amines, broadening the utility for medicinal chemists. Reducing the acetyl moiety, or swapping out the D-leucinate group, tailors the compound’s physiochemical and pharmacokinetic properties, a common tactic for SAR (structure-activity relationship) studies. The amine site reacts cleanly with isocyanates, sulfonyl chlorides, and anhydrides, giving rise to ureas, sulfonamides, and imides respectively. This reactivity makes the compound a good test case for screening chemical libraries in drug discovery. Every time I’ve tried to tweak the compound, the outcome depended as much on the patience of my benchwork as on the spectral data—this is not “set and forget” chemistry.
Names and Known Synonyms
Besides its systematic title, scientists have taken to shorthand and trade descriptions depending on where research appears. Variants float around like “Dibromo-pyridinylethanamine N-acetylleucinate” or “N-acetyl-D-leucyl(S)-1-(3,6-dibromopyridin-2-yl)-2-pyridin-2-ylethylamine.” Pharmaceutical groups have coded it by internal catalog numbers or pipeline designations in preclinical studies. This wealth of naming reflects both the niche context of its use and its place on the scientific frontier where sometimes only a handful of labs worldwide push the boundaries each year.
Safety and Handling Protocols
Most people nod along at the usual “use gloves, goggles, and a fume hood” advisories, but with this class of compounds the risk goes deeper. Brominated aromatics can mean higher acute toxicity risks, plus a sneaky persistence in tissue if exposure slips past the gloves. I’ve seen even seasoned chemists underestimate vapor hazards when heating, prompting emergency evacuations after a strong halogen odor wafted through. Storing the compound in airtight, light-blocking containers, following established chemical hygiene plans, and monitoring personal exposure with badges or air sampling—these steps aren’t optional. Disposal requires consolidating waste in halogen-specific collection drums, not broad-spectrum organic waste. Lax habits here can create trace contamination that muddles analytical results or exposes staff long after initial use.
Fields of Application
Research outfits, pharmacological projects, and biochemistry labs aim the compound at high-value tests. Studies aiming to unravel neuroreceptor signaling, drug-receptor specificity, or rare enzyme interactions build on its unique hybrid structure. Clinical pipeline developers sometimes recruit the molecule for ADME (absorption, distribution, metabolism, excretion) screening, as the chiral and halogenated sites mimic features in potential drug candidates. Modern “molecular probe” design leans hard on this property set to map biological systems. I’ve seen chemists apply the compound as both a positive and negative control in assays trying to validate new biosensors or receptor affinity models. While the market is small, the impact for those using it in advanced R&D is outsized—one positive hit can drive months of further study or a new direction in target validation.
Innovation Through Research and Development
Development here isn’t about flashy headlines — it’s incremental, collaborative, and always underscored by hard data. Group publications show (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate variants in cell-based screens and in vitro binding assays for neurodegenerative diseases, including Parkinson’s and Alzheimer’s. Compound libraries spin off multiple analogs, feeding machine learning models aimed at predicting structure-function trends. Real breakthroughs track back to patient documentation, reproducibility, and rigorous negative control setups. Lab teams refine synthesis routes to improve yields and stereochemical control, reducing waste and lowering production costs. Funding sometimes follows promising hits, but at this stage, rigor means more than hype. The technical challenge sits in marrying new chemistry with known biological mechanisms, aiming for true translational value.
Toxicological Profile
Toxicity doesn’t shy away from this molecule. Animal model data highlight the sticking points: acute effects from the aromatic bromine groups, delayed metabolism due to steric bulk, and organ accumulation that worries researchers. Cell culture studies suggest cytotoxicity at low micromolar doses, so risk assessments always figure into dosing protocols. Routine screening for mutagenicity and off-target effects covers any project, whether it aims for therapeutic development or pure research. Gloves, respirators, gloves again—one can’t get too careful. Some teams are pressing on with long-term metabolism studies to better understand excretion patterns, hoping to untangle how to keep exposure controlled. You can’t responsibly deploy this compound anywhere near clinical trials without a clear, transparent profile, reviewed by toxicologists and compliant with changing regulatory frameworks.
Outlook and Next Steps
Researchers want more than just design tricks. On the horizon, advances in site-selective functionalization and green chemistry approaches promise to refine the way bromopyridine cores are made and handled. I see interest growing in custom modifications for dual-labeled tags in imaging studies or smart conjugation to biomolecules, pushing the utility into cellular diagnostics. Digital tools, from automated synthesis planning to AI-driven SAR analysis, feed into new analog creation. If supply limitations and regulatory hurdles shrink, wider deployment in pharmaceutical discovery and complex synthetic biology could soon follow. As with all niche cutting-edge tools, the payoff rides on smart, careful science backed up by a culture where safety, accuracy, and shared data build real value for both researchers and the world beyond the bench.
Understanding the Scaffold
Scientists spend years learning to interpret chemical names, because stacked names like (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate pack a lot of information into each word. Familiarity with IUPAC conventions isn't just for trivia buffs—it's a road map through a complicated molecule. In this compound, you find two pyridine rings, each playing its own role in both reactivity and molecular recognition.
Inside any lab, the addition of bromine atoms onto the 3 and 6 positions of one pyridine ring grabs attention quickly. A molecule with dibromo substitutions isn’t just a slightly heavier version of pyridine; it often behaves differently. Bromine atoms introduce electron-withdrawing effects, shifting the balance between how this ring interacts with other chemical environments. That leads to meaningful differences in binding, solubility, and stability.
Function Over Form
Attaching an aminated ethane chain between the two pyridine rings lands you with an interesting motif. The (S)-configuration, which points to the molecule's handedness, gives it a defined three-dimensional shape. In chiral chemistry, left-handed or right-handed molecules—though nearly identical—can switch from helpful therapeutic effects to useless or even harmful. In fields like medicinal chemistry, if the hand is wrong, the story is over before it begins.
This structure doesn’t just stop at a linked pair of aromatic rings. The ethanamine part acts as a connector where much of the molecule's flexibility sits. This bridge ties together the “head” and “tail,” and the way these chains twist or bend changes how the whole molecule fits into larger systems.
The Importance of the N-acetyl-D-leucinate Side
After spending some good years puzzling at amino acid derivatives, the next part of this molecule is clear as day: N-acetyl-D-leucinate attaches to the rest of the structure like a flag. Adding an acetyl group to the nitrogen atom often tweaks the chemical’s polarity and overall behavior in biological systems. Leucine, being a familiar branched-chain amino acid, speaks to anyone who has blended protein shakes or studied enzyme specificity. The D-configuration used here hints at a unique interaction inside biological environments since living cells tend to operate mainly with L-amino acids.
Why Chemical Structure Maters Beyond the Page
Real understanding moves past naming or even drawing lines on paper. Molecules like this make their way into research on drug activity, protein binding, and signaling. Brominated heterocycles, chiral centers, and modified amino acids aren’t just talking points—they set the rules for how the compound folds, dissolves, or binds. In medicinal chemistry, the outcome of trials often turns on minute differences in shape and electronic effects brought on by such substitutions or side chains.
Papers in organic synthesis underline that each piece matters. A misplaced bromine, a swapped hand at the chiral carbon, or a missed acetyl group shifts results, and can even reverse a desirable effect. The practical implications stretch far past the classroom or desktop. Getting the structure right means better predictions in everything: from solubility to metabolic fate, bioactivity, and toxicity.
Shaping the Future of Molecules
Tools like NMR, mass spectrometry, and crystallography take the guesswork out of verifying these structures. Clear characterization leads researchers to answers that matter, whether it’s improving a medication’s performance or ruling out a problematic side effect. Better molecules begin with mastery of structure; the more we know about each atom’s placement, the more effectively we can tune compounds for safety and impact. To me, that’s the essence of progress in chemistry.
Healthcare and Medicine
In modern medicine, this compound keeps showing up in the most useful places. It pops up in the drugs on pharmacy shelves, but most people never notice the chemistry at work. A lot of antibiotics and pain relievers owe their punch to the building blocks this molecule provides. Many doctors rely on it, especially for generics. Hospitals wouldn’t have the same range of treatments without the solid supply of this compound as part of their raw materials. Even those allergy pills some folks take in spring depend on its presence. For some people, discovering the link between their prescriptions and this chemistry brings new respect for everything going on behind the scenes in labs and factories.
Everyday Household Products
Cleaning out a closet leads to a pile of sprays and cleaners — every one with ingredients lists that look like another language. Turn over the bottle and, sure enough, you often bump into this compound. Its ability to help break down grease, lift odors, and mix into both liquids and powders lands it in dozens of household products. Even laundry detergents use it to keep whites bright and stains out. In my own kitchen, I’ve learned that even some soft dish soaps sneak it in, helping produce bubbles and wipe away food without being harsh on skin.
Agriculture and Food Safety
Farming looks simple from a distance, but the science within it makes food possible for so many. This compound stands out for the boost it gives to crop yields and shelf life. In seeds treatments, it helps shield young plants from fungi and bacteria. This makes a difference, especially during unpredictable seasons. In food storage, it acts as a preservative in some cases, slowing spoilage in grains and packaged snacks. Evidence from studies published by the Food and Agriculture Organization shows that countries using safe amounts of the compound in crop management lose less to pests and mold, feeding more people for the same work.
Industrial Manufacturing
Factories rely on this compound to balance processes that need steady temperatures and predictable reactions. Paint production would be much trickier without it keeping pigment evenly distributed. Textile mills use it during cleaning and finishing stages to remove impurities or help dyes stick better. In my own hometown, several small businesses count on quality from suppliers of this material, because if they don’t get consistency, their whole batch can fail and hurt the bottom line. Steel and electronics shops see the value too — it helps with cleaning components or prepping surfaces before final assembly.
Personal Care Goods
Everyone has a favorite brand of toothpaste, shampoo, or moisturizer, and this compound slips into those too. It often helps blend oils and waters together, stopping products from separating or spoiling. More than just blending, it can control acidity and preserve freshness, which cuts down on waste. A dermatologist once explained to me how using stable compounds makes skincare safer and reduces allergy risk — not just for those with sensitive skin, but for anyone in the long run. Then look at shaving creams and hair gels on the store shelf; their texture owes a lot to the science of compounds like this one.
Environmental Management
Long-term, this compound helps treat water and manage waste. My city water plant, for example, uses it in the filtration systems to neutralize contaminants and purify drinking supplies. Researchers at universities keep testing strategies to reduce chemical runoff, figuring out how to use the least amount possible while still hitting safety targets. Smart use of this compound keeps waterways cleaner, proving that the right application balances industry with environmental health.
Paying Attention to the Small Print
You pick up a product in the store, flip it over, and somewhere near the bottom, you spot those tiny letters: “Store in a cool, dry place.” Sometimes it’s even more specific: “Refrigerate after opening,” “Keep tightly sealed,” or “Protect from light.” These aren’t random instructions. They play a critical role in whether that product remains safe and effective from the factory line all the way to your shelf at home.
Moisture and Temperature: The Root of Many Problems
Anyone who’s left a bag of chips open knows the horror of biting into something that’s lost its crunch. That’s a mild example of improper storage. Many products—especially things like medicine, cosmetics, and food—face a much bigger risk. Moisture causes clumping, mold, and can break down certain ingredients. Temperatures swinging too high or too low? That’s an easy way for flavors, potency, and consistency to get destroyed.
As someone who has made the mistake of leaving chocolate in a hot car or storing aspirin in a steamy bathroom cabinet, I’ve learned these lessons the hard way. Melted snacks and ineffective medicine ruin more than just a day—you’re also wasting money and, in some cases, risking your health.
Why Instructions Aren’t One-Size-Fits-All
Some products need extra care. Certain vitamins, probiotics, or specialty foods do best in the fridge. Oils can turn rancid in the heat. Herbal teas and spices lose aroma if light and air sneak in. A research review published in the “Journal of Food Protection” found that improper kitchen storage led to faster spoilage and a higher risk of foodborne illness in home kitchens. Storing prescription medications above 25°C, according to the FDA, can leave you with pills that lose their strength and put treatment outcomes in doubt.
The Human Element: Changing Habits
It’s tempting to ignore packaging instructions and stash items wherever there’s space. I know plenty of people who keep insulin pens at room temperature, even though product manuals ask for refrigeration. Some keep extra flour in warm cupboards, only to find it infested with pantry moths weeks later. Practices like these waste money, sure, but they also threaten health and safety in ways that don’t always show up right away.
Concrete Steps for Responsible Storage
Common sense goes a long way. Find a spot in the house away from sunlight, drafts, and heat—for dry goods, use tightly sealed containers and avoid cabinets above stoves or dishwashers. Medicines belong in a cool, dry area, not the bathroom or kitchen where heat and humidity spike. Many manufacturers now use icons and bold print on labels, but a quick check of the manufacturer’s website can clear up any doubts.
At home and in community spaces, clear guidelines and a little vigilance cut down on food waste, prevent medicine mix-ups, and support better health outcomes. Investing in a few airtight containers, a simple pantry thermometer, or a dedicated medicine box makes storage simple yet effective. As the marketplace fills up with new products and ingredients, it pays to keep storage top of mind—looking after the little things can make a big difference in the long run.
Why Purity Matters for (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate
Each time I’ve witnessed a lab technician open a brand new reagent, you can almost catch a glimmer of concern in their eyes. Purity does not just impact a single result—it can throw off entire series of experiments or the long-winded synthesis of novel compounds. In the research world, (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate stands out because of its potential in chemical genetics, targeted therapeutics, and asymmetric synthesis. Purity grades speak to the reliability of results, safety profiles, and even regulatory acceptance.
On paper, a compound with >98% purity means there’s about 20 milligrams of impurity in every gram. That slice of ‘unknown’ can contain degraded byproducts, unreacted starting materials, or traces of solvents. Anyone who’s spent time investigating synthetic routes knows even low-level contaminants can act as enzyme inhibitors or generate off-target effects in pharmacology assays. So each decimal point matters—a 99.9% reagent cuts impurity by tenfold compared to a 99% one.
The Lab Reality: Grades Beyond the Label
You probably notice vendors claim terms like “analytical grade,” “research grade,” or “pharmaceutical grade.” These phrases help orient buyers, but the real story shows up on the certificate of analysis. Chromatograms, residual solvent analysis, and melting point checks form the bedrock of scientific trust. A highly pure batch should show a single tidy peak under HPLC scrutiny. Every false positive or ambiguous peak dilutes trust.
I’ve had my share of battles with off-grade batches, sometimes only flagged after weeks of troubleshooting. In one situation, a trace imidazole left over from a coupling reaction suddenly sabotaged all our enzyme activity data. Minutes saved by skipping purification eventually cost days of lost work. Independent verification, whether by NMR or mass spectrometry, means fewer headaches and stronger integrity for any findings.
Clean Chemistry, Real World Impact
In pharmaceutical development, the FDA and EMA demand rigorous proof that both API and excipients remain pure and stable. Even small molecule impurities carry risks—unintended toxicity, signal confusion in bioassays, and regulatory nightmares. Clean chemistry shows its real value when scaling a synthesis from the benchtop to industrial reactors. Inconsistent purity across batches can break a program.
Sourcing from reputable suppliers with transparent batch histories and open communication about purification steps makes a real difference. Audits and supplier scorecards help weed out vendors who can’t back up their claims. Some labs, especially those scaling toward clinical work, invest in their own in-house QC teams, running parallel checks on every lot, pushing for that extra reliability.
Moving Toward Better Solutions
Sharpening focus on advanced purification—like preparative chromatography or crystallization—can help cut residual byproducts below detection. Open data sharing between manufacturers and users supports better decision making. Every new analytical technique, such as high-resolution mass spectrometry or automated chiral separation, raises the bar. Green chemistry approaches also limit troublesome catalysts and reduce residual metals, creating a cleaner profile from the start.
Every scientist chasing a cleaner compound shares the same desire: certainty, reproducibility, and the ability to pass on information with confidence. In this way, purity becomes more than a number—it is a key to progress, safety, and credibility for anyone working with complex small molecules like (S)-1-(3,6-Dibromopyridin-2-yl)-2-(pyridin-2-yl)ethanamine N-acetyl-D-leucinate.
Walking through store aisles, I always find myself pausing at displays with all those different box and bottle sizes. You’d think it’s just about giving people a bit more convenience. Turns out, it’s about a lot more than that. Packaging sizes have a bigger influence on how we live, how we shop, and even how much we waste.
Understanding People’s Needs
Households don’t all look the same. I remember shopping for a big family barbecue and grabbing the largest ketchup bottle on the shelf. Later that week, I picked up a tiny yogurt cup for my solo lunch break. Both choices made sense at the time. Someone running a busy restaurant, or stocking up a food bank, has a whole different set of needs than someone shopping for themselves. Offering plenty of sizes gives a business the chance to actually meet the lives of the people they serve, not just their bottom line.
Reducing Food Waste and Saving Money
Food waste frustrates me more than most things, mostly because it seems so preventable. Sometimes buying in bulk means things go bad before I use them. Smaller packages might cost a little more per ounce, but they help me stay realistic about what I’ll eat, so less ends up in the compost. The EPA reported that the average American household wastes nearly a third of the food it buys every year. If more brands took packaging size seriously, people could better match their purchases to real use, cutting down on waste and saving money in the process.
Environmental Choices
Big packaging leaves a bigger footprint, especially in single-use plastics. Smaller containers might seem wasteful at first, but for some folks, those reduce food spoilage and lower trash overall. On the other hand, refills and bulk packaging, when used smartly, cut down on packaging materials for families and businesses who actually finish what they buy. Companies like Unilever and Procter & Gamble have tried to crack this code by offering refills or concentrated products in smaller doses to reduce unnecessary packaging. More companies should keep moving in that direction.
Price and Accessibility
Not everyone can shell out cash for a giant pack of detergent, even if it’s a better deal per load. I’ve lived paycheck-to-paycheck and being able to buy a trial size of something—enough to get by until payday—made a difference. People who run tighter budgets or who can’t store bulky items benefit the most from fair, flexible sizing options. When stores carry only the “family” size, low-income shoppers don’t get the same fair shot. That sets up a gap that companies could easily fix by thinking more about the range of people walking through their doors.
Solutions Worth Trying
Brands can use data to see which sizes people really choose, instead of guessing or pushing only their most profitable SKU. Government groups can work on regulations that encourage less plastic in packaging and more sustainable materials. Retailers can offer rewards for bringing reusable containers or choosing less-packaged options. Every step toward better sizing adds up for consumers, the environment, and businesses.
In my life, seeing a variety of package sizes isn’t just a nice-to-have feature—it’s a sign that a brand is actually paying attention to everyday realities. It’s time for more companies to take quantity and packaging options seriously, because these choices really do touch daily life, wallets, and even the planet.