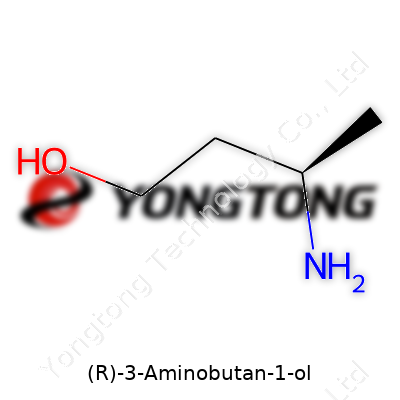
(R)-3-Aminobutan-1-ol: A Deep Dive Commentary
Historical Development
Back in the late 1900s, chemists needed reliable chiral building blocks for pharmaceuticals. (R)-3-Aminobutan-1-ol, a small chiral amino alcohol, turned out to be one of these go-to molecules. Its story began among the efforts to synthesize beta-blockers and other asymmetric compounds, as researchers realized that a good chiral source could change the way drugs interact with the body. My early work in a university lab introduced me to the tedious separation of enantiomers, and finding (R)-3-Aminobutan-1-ol on a reagent shelf meant less pain, more precision. The demand for purity and enantiomeric excess in the pharmaceutical world shaped the commercial production methods, pushing manufacturers to improve both stereoselectivity and yield. These improvements didn’t happen overnight—years of optimization and plenty of trial-and-error allowed for more accessible and safer drug development worldwide.
Product Overview
To the layperson, (R)-3-Aminobutan-1-ol may sound obscure, but its presence in labs underscores its importance in modern chemistry. This colorless liquid, carrying an amine group and a primary alcohol, often finds a place in synthesizing active pharmaceutical ingredients. Far from just a building block, its chiral center brings unique reactivity, which organic chemists value for constructing tailor-made molecular frameworks. Companies label it for research, development, and use in routes leading to medicines targeting everything from heart conditions to neurological disorders.
Physical & Chemical Properties
Measured properties of (R)-3-Aminobutan-1-ol reflect its dual chemical nature. It’s a clear liquid at room temperature, though subject to minor discoloration with age. With a boiling point near 170°C and a melting point well below room temperature, it pours easily but needs careful storage. The presence of both an amine and an alcohol makes it versatile but also reactive with acyl chlorides, aldehydes, and acid anhydrides. Its refractive index and specific rotation became guideposts for checking purity and chiral excess. A faint amine smell gives away its classification, and it dissolves in water and polar organics, making it especially workable for lab chemists.
Technical Specifications & Labeling
Most suppliers set strict guidelines for purity—above 98%—and low water content. The product’s typical labeling includes stereochemistry, CAS number, lot number, and expiration date, allowing full traceability from synthesis to storage and final use. Each bottle ships with a certificate of analysis for both chemical and optical purity, many going further to specify how much of the unwanted enantiomer remains. Chemical supply houses warn about moisture sensitivity and volatility under certain storage conditions, so a dry, cool place is standard advice.
Preparation Method
Synthesizing (R)-3-Aminobutan-1-ol centers on choosing the right starting materials to guarantee stereochemical purity. Asymmetric synthesis usually starts with an α-ketoester and employs chiral catalysts or auxiliaries to tip the scales toward the (R) configuration. Another frequent industrial route involves enzymatic reduction, which produces a high enantiomeric excess with minimal byproducts—good for the environment and easier to scale. My time spent troubleshooting these syntheses revealed that high selectivity comes at the price of longer process development and strict control of temperature, solvent, and reagent purity.
Chemical Reactions & Modifications
This molecule’s two main functional groups open up a range of modifications. Alkylation or acylation at the nitrogen generates derivatives with altered basicity, which pharmacologists use to adjust drug absorption or blood-brain barrier penetration. Oxidation of the alcohol moiety leads to amino acids, often exploited in peptide synthesis. I’ve watched my colleagues, especially in medicinal chemistry teams, find new routes to inhibitors and agonists by simply tweaking the substitution pattern on the aminobutanol core. Its structure invites creativity, making it a kind of chemical “Swiss army knife” in the lab.
Synonyms & Product Names
(R)-3-Aminobutan-1-ol goes under several names: (R)-3-Amino-1-butanol, (R)-1-Butanol-3-amine, and (R)-3-Aminobutanol. Commercial catalogs sometimes shorten it to (R)-3-ABOL. The variety of synonyms can confuse, especially for those jumping between manufacturers or reading multilingual literature. It always pays to cross-check CAS numbers (107667-99-6 for this enantiomer), as chemistry journals and regulatory filings refer to many compounds with multiple names.
Safety & Operational Standards
Working with (R)-3-Aminobutan-1-ol calls for attention to both chemical hazards and best lab practice. Skin and eye irritation can result from even brief contact, so gloves and goggles are standard protocol. Ventilation systems or properly maintained fume hoods keep vapors out of breathing zones. Spills need prompt cleanup with water; the substance can become slippery, and improper disposal leads to regulatory headaches due to the primary amine content. I always keep safety data sheets on hand, as even a simple spill can mean trouble when working on a scale-up batch. Regulatory standards set by agencies like OSHA and REACH keep safety measures current and comprehensive.
Application Area
This amino alcohol appears in a variety of chemical, pharmaceutical, and even agricultural syntheses. Drug developers rely on its chiral nature for efficient asymmetric synthesis, especially where beta-blockers or anti-viral agents need a non-racemic intermediate. Diagnostic reagent producers use it to build probes for medical imaging. Its presence also shows up in research papers focused on catalysis, where it acts as a ligand or chiral modifier. Several agricultural chemists experiment with its derivatives for crop protection, and industrial chemists sometimes use it in specialized resin formulations.
Research & Development
R&D programs targeting (R)-3-Aminobutan-1-ol focus on sustainability, atom economy, and greener reaction conditions. As someone who’s worked in process optimization teams, I have seen pressure mounting for enzymatic transformations that cut down on hazardous waste. Automated continuous-flow systems now make reproducible synthesis simpler, while in-line monitoring with NMR or HPLC lets teams catch errors instantly. Academics keep pushing the frontiers—there are always new chiral ligands, or creative modifications of this scaffold, appearing in the literature to improve selectivity and biological compatibility for the next wave of pharmaceuticals.
Toxicity Research
Exposure data for (R)-3-Aminobutan-1-ol remain somewhat limited compared to old-world amines. Standard toxicity assays in rodents haven’t revealed major red flags, though acute inhalation and ingestion risk remains. Studies show moderate irritation on skin and mucosal tissues, and environmental persistence stays low due to its biodegradability. Chronic data remain scarce, underlining the need for ongoing attention, particularly as it moves toward higher volumes in pharmaceutical pipelines. Toxicologists in my network stress that safety guidelines should remain conservative until more long-term exposure studies get published.
Future Prospects
The horizon looks busy for (R)-3-Aminobutan-1-ol. Specialty synthesis trends, combined with the explosive growth of chiral drugs and biologically active molecules, guarantee ongoing demand. Greener manufacturing, especially biocatalytic processes, holds special promise in cutting costs and reducing environmental impact. My guess is that pharmaceutical companies will increasingly see (R)-3-Aminobutan-1-ol not just as a reagent but as a platform molecule, with potential in new therapeutic classes, advanced diagnostics, and beyond. Research into analogs and more sustainable synthetic routes continues, ensuring it stays relevant as both science and regulations evolve.
Why Chemists Reach For (R)-3-Aminobutan-1-ol
Walking through any research lab or pharmaceutical production line, you’ll probably find (R)-3-Aminobutan-1-ol somewhere on the shelf. Scientists prize it for its ability to serve as a building block in the synthesis of several antiviral and cardiovascular drugs. Take tenofovir, a backbone in HIV therapy — the making of its active form depends on smart use of this molecule. Many chemists remember the headaches when searching for chiral amino alcohols, especially those with high purity. Racemic mixtures often send yields and outcomes in the wrong direction, so having a reliable source of (R)-3-Aminobutan-1-ol in a well-defined enantiomer makes a real difference.
More Than Just a Building Block
Think of (R)-3-Aminobutan-1-ol like a Swiss Army knife — its structure supports several applications. Drug makers use it when searching for ways to improve molecular activity or reduce unwanted side effects. Its chiral center plays a part in how drugs interact with enzymes and receptors. I’ve seen chemists get excited after a simple switch in chirality led to less toxicity and better outcomes in animal studies.
Not every pharmaceutical synthesis looks straightforward. Some compounds include subtle structural demands that only specific amino alcohols can satisfy. In my own work with startup formulators, I noticed how the market for HIV drugs and certain heart medicines relies on the predictable reactivity of this molecule. This fact isn’t just trivia — consistent access to quality (R)-3-Aminobutan-1-ol can speed up development pipelines, lower costs, and potentially reach patients sooner.
Supporting Modern Lab Culture
Every project in the lab juggles budgets, timetables, and regulatory reviews. Supply issues with a small component like (R)-3-Aminobutan-1-ol can sideline entire drug programs. Reliability here means more than chemistry — it contributes to lab morale and trust between research teams. Some of my most rewarding collaborations grew from a shared appreciation for careful sourcing and planning, especially since contamination or low purity in small-molecule ingredients can derail expensive batches of final product.
Specialty chemical suppliers pay close attention to the demands that come from both pharma giants and smaller research outfits. Their focus on producing high-purity enantiomers like (R)-3-Aminobutan-1-ol means chemistry teams spend less time troubleshooting and more time pushing the science forward. My colleagues often talk about this molecule’s reputation — it’s trusted, versatile, even crucial for a surprising number of patented products.
Challenges and Opportunities Ahead
Sourcing reliably hasn’t always been easy, especially in years when global events caused serious shipping disruptions. Companies notice which suppliers stay true during shortages, and the best ones open their QC data for inspection. There’s a push for even more sustainable and cost-effective routes to making chiral building blocks like (R)-3-Aminobutan-1-ol — catalytic asymmetric synthesis, greener solvents, and smarter recycling methods are hot topics at conferences.
A handful of start-ups now tailor production lines to pharmaceutical needs, decreasing impurities and offering documentation upfront. Partnerships between pharma and specialty chemical brands create more transparency, so both sides plan confidently. Regular audits and cross-lab verification keep standards high, while emerging techniques in stereoselective synthesis offer hope for even better access and lower prices.
For anyone in the fields of medicinal chemistry, drug discovery, or pharma manufacturing, (R)-3-Aminobutan-1-ol stands out for its proven record. It connects innovative science to tangible patient health, proving that even the smallest molecules can drive enormous change.
Chemical Structure Breakdown
Peeking into the structure of (R)-3-Aminobutan-1-ol, you’re looking at a molecule with the formula C4H11NO. Its scaffold has a four-carbon chain, with an amino group on the third carbon and a hydroxyl group at the tip, connected to carbon one. For chemists and folks working in pharmaceutical research, this kind of configuration is far from arbitrary. Nature cares a lot about the way molecules twist and turn, and (R)-3-Aminobutan-1-ol shows that in its (R) stereoisomeric form. Here, “R” isn’t just a random label—it marks the direction the molecule’s atoms lean, giving it a specific biological fingerprint.
Looking closer, you find the chiral center at the third carbon, attached to the amino group (NH2). The “R” means the priority of groups spirals to the right, as seen in the Cahn–Ingold–Prelog rules. Put another way: the chain starts at a terminal alcohol (–OH) at carbon one, switches track across two more carbons, and then finishes at a junction bearing (NH2), a hydrogen, and a methyl group. Picture a tangle of spaghetti where that one distinct loop makes all the difference.
Why Stereochemistry Matters in Practical Life
Chemistry isn’t just about atoms stuck together in a row. It’s about outcomes—how those shapes and arrangements give us molecules that act in precise ways. For example, swap the orientation of an amino group or alcohol and you can land with wildly different pharmacological effects or lose activity altogether. The (R) configuration often sets the tone for biological interactions, from enzyme binding to metabolic fate.
Anyone who’s ever cracked open a drug chemistry textbook will know the trouble that arises from ignoring chirality. Medicines like thalidomide, which had tragic consequences after its isomers weren’t properly distinguished, offer a lesson most scientists carry forward. That’s why current labs check and double-check chirality, especially with molecules like (R)-3-Aminobutan-1-ol that might become stepping stones to larger, more active pharmaceutical compounds.
Real-World Uses and Solutions
You spot (R)-3-Aminobutan-1-ol in chemical libraries geared toward asymmetric synthesis. Its shape helps build other molecules that demand specific orientation. Take pharmacology—the building blocks of a drug can only lock on to receptors if their hands and feet point the right way. Industry doesn’t just churn out these molecules; experienced chemists coax them into exactly the form they need. Sometimes, factories use chiral catalysts or enzymes to tilt the balance, making sure only the right version slides off the production line.
The production of pure (R)-enantiomer calls for shrewd methods. Companies lean on chiral catalysts and biotransformations. Enzyme-catalyzed synthesis proves reliable, since enzymes hold a steady hand in steering reactions with pinpoint accuracy. Big pharma isn’t shy about using these bio-inspired tools. Scaling up such specialized reactions raises roadblocks but also encourages clever engineering and new tech development.
Looking Ahead
Precision in the shape and form of molecules like (R)-3-Aminobutan-1-ol doesn’t just serve high-end research. It trickles down, touching medicine safety, manufacturing quality, and even regulatory policy. As more therapies depend on chiral purity, the savvier the processes have to become. Better catalysts, efficient separation methods, and smarter analytics wait on the horizon, driven by real-world needs—not just theory. Deep understanding of molecules at this granular level powers safe innovation, underlining how far a “simple” alcohol–amine can take us.
Why Storage Conditions Matter
Labs often handle chemicals that react to changes in their surroundings. Keeping (R)-3-Aminobutan-1-ol in top shape means paying attention to where and how it sits on the shelf. Temperature, light, air, and contamination build the foundation of chemical safety, but real-world examples show difficulties pile up fast when routines slip. A few degrees too warm or a leaky cap can turn years of careful work into waste.
Temperature: Don’t Overlook the Obvious
Most chemists and drug developers keep (R)-3-Aminobutan-1-ol in the fridge, usually at 2–8°C. This slows down possible degradation or unwanted reactions. Stashing it at room temperature leads to headaches later. I remember rushing an experiment only to realize the sample degraded since it sat on the bench for a few extra days. Even if it doesn’t decompose quickly, chemical purity drifts, and that taints reliability and reproducibility.
The Air Factor
Air and moisture, invisible most days, change the fate of sensitive amino alcohols. I’ve seen glassware sweat if left open in a humid lab, and over time, the powder or liquid can absorb water. This changes weight, throws off measurements, and risks side reactions. After exposure, a pure compound won’t respond the same way in synthesis. Tight caps, nitrogen blankets, or desiccators block the worst offenders. Routine matters; don’t skip the extra step of sealing bottles right away.
Light Sensitivity
Shining exposure to strong light sometimes causes breakdown of certain molecules. Dark bottles aren’t just for show; they actually limit light-triggered changes. If you spot a supplier shipping (R)-3-Aminobutan-1-ol in clear bottles, question their standards. Amber bottles set the baseline in every trustworthy lab. Store the bottle deep in a closed cabinet to keep daylight away, especially for long-term storage.
Contamination Sneaks In
Rushing through the weighing process lets dust or skin oils drop in. Take precautions—clean spatulas, gloves, and never double-dipping into the stock bottle. Even a grain of old powder from an unclean spoon sets off unexpected results, especially for researchers who depend on ultra-pure starting material. I’ve watched colleagues frustrated by odd peaks in chromatograms that traced back to sloppy techniques during storage or sample return.
Disposal and Safety
Respecting safe storage habits naturally lowers risks during disposal or emergency leaks. Proper labels, clear hazard warnings, and storing away from acids or oxidizers shield everyone in the workspace. Fire codes and good documentation do more than tick boxes—they protect the people putting their health on the line day after day. Odor, spills, or label damage always signal it’s time to call the safety officer.
Solutions Going Forward
Getting chemical storage right means clear training for new staff, regular audits of old stock, and investing in fridges with lockable doors. Building a culture where reporting temperature swings or faulty caps is routine creates safer workspaces. Trust grows when people know the rules aren’t just ink on a policy. Science isn’t just about clever theories—it’s about careful practice, bottle after bottle, day after day.
Real Concerns in Purity
Most people never hear about (R)-3-Aminobutan-1-ol until it pops up in a lab request or a pharma formula. For chemists, quality can make or break a batch, a trial, or an entire synthesis. Let’s be clear: purity isn’t an abstract promise printed on a label. Every digit after that decimal separates a clean run from a day ruined by noisy chromatograms or botched results.
I once stumbled over an order where the same compound came in two “types”— and the difference was more than a price tag. The technical grade bottle delivered a nasty byproduct I didn’t catch until late in the process. The higher-purity batch sailed right through. Here’s the thing—most reputable suppliers know that researchers want options, so they stock this building block in multiple purities: technical, laboratory, and research/analytical.
Why the Numbers Matter
No two syntheses ever tolerate the same level of “impurity.” The fine chemical world loves numbers: 95%, 97%, 99%. Tiny changes in those numbers can bring big shifts in solubility, reactivity, even storage stability. If you’re in medicinal chemistry, you need at least 98% purity just to avoid troublesome signals in NMR or LC-MS. Process chemists aiming for scale find gross errors if their raw material is just 95% pure. The “rest” can be water, other isomers, or unholy side products that poison subsequent steps.
If companies see a steady market for a compound, they’ll refine it into multiple grades. But that only goes so far because cost and demand play referee. Sometimes only technical or lab grade sits on the shelf. Ultra-pure forms get expensive, and not every process needs that cost. It’s frustrating when a catalog lists only one grade, while another supplier offers two or three. A wider selection means more flexibility, less wasted time.
Quality Over Labels
Certificates of Analysis (CoA) tell the real story. Serious labs ask for them, cross-check against the numbers, and check impurity profiles—not just “purity by HPLC” but also moisture and other specs. I’ve handled CoAs from tiny startups and giants. Sometimes, the surprises come from the smallest print. That’s where a chemist finds solvent residues, enantiomeric ratios, or metals that sneak through early steps. This detail matters just as much as gross purity, especially in pharma and diagnostics.
Customers shouldn’t get trapped by nice-sounding names. “Analytical grade” might mean something for one supplier, something else for another. I recommend always demanding the CoA, talking to the technical team, and checking for unusual ions or signals before signing off.
Can We Improve Supply Chains?
There’s room for better transparency. If more companies published detailed impurity profiles online, labs would save days chasing after data. Right now, it’s still mostly the best-funded organizations that can get custom purities or tighter specs. Affordable, consistent high-purity batches would help startups and universities take bigger chemical risks—without gambling on someone else’s leftover contaminants.
Until supply chains catch up, the best practice means calling vendors, comparing documents, and treating “purity” as more than a marketing line. Behind every number is real work, real risk, and a need for experiments that actually deliver what the data promises.
Understanding the Risks
Handling chemicals in any laboratory or industrial setting pushes for care and respect. (R)-3-Aminobutan-1-ol brings its own set of challenges, so taking clear steps to keep people and workspaces safe matters. I remember my first morning training in a research lab, my mentor reminded me: experience isn’t enough—every vial, no matter how common, deserves attention.
Personal Protective Equipment: First Line of Defense
Gloves come first when working with (R)-3-Aminobutan-1-ol. Nitrile gloves tend to offer solid resistance to most organic compounds. A well-fitting lab coat and chemical splash goggles should always be worn, especially if there’s potential for splashing. Simple glasses don’t cut it. Face shields help in larger-scale operations, which makes sense when spills become costlier—not just in money, but in health.
Ventilation and Workspace Setup
Fume hoods go a long way to keep airborne exposure down. With (R)-3-Aminobutan-1-ol, protection from vapors and aerosols matters, since inhaling any low-molecular-weight amine can create discomfort or health complications. I’ve seen labs retrofitted just to improve airflow, and the difference in comfort—and safety—was obvious. Keep benchtops uncluttered, with spill trays to contain leaks.
Safe Storage and Labeling
A chemical like (R)-3-Aminobutan-1-ol belongs in tight, clearly labeled containers. Storage areas should stay cool and dry, away from heat sources and incompatible reagents like strong acids or oxidizers. Some folks overlook labeling because they ‘know what’s inside’ but one wrong assumption can spark confusion or worse, an accident. Even seasoned colleagues make mistakes—good labeling keeps everyone honest.
Spill and Exposure Response
During a research project last year, a small spill of an amine turned an ordinary afternoon into a lesson on readiness. Absorb small spills with inert materials, then collect waste properly for disposal. Large spills should drive people from the area until air clears and professional cleanup arrives. In any contact with skin or eyes, rinse for fifteen minutes under running water. If inhaled, seek fresh air right away, and see a healthcare provider. These aren’t steps to rush.
Training and Communication
No matter how experienced the crew, regular safety briefings help everyone remember proper handling—complacency leads to broken routines. Reading an SDS before starting work gives a clear idea of the compound’s hazards. Good labs keep safety data sheets accessible on every bench, updated as compounds get rotated or replaced. Quick reminders—where safety showers are located, how to reach emergency contacts—can be the difference between a scare and a story.
Waste Disposal and Environmental Concerns
Disposing of unwanted (R)-3-Aminobutan-1-ol wastes requires sealed, labeled containers and a trusted hazardous waste service. Some solvents turn dangerous if poured down the drain. Chemical stewardship extends beyond the lab; responsible teams avoid shortcuts, protecting groundwater and the wider community.
Building a Culture of Safety
Real safety grows from consistency and looking out for colleagues. Sometimes the simple act of reminding a distracted coworker to wear their goggles keeps a minor mistake from escalating. Open discussions about near-misses or problems encourage everyone to speak up. In my experience, the safest workplaces don’t just teach the rules—they empower every person in the room to own them.