(R)-2-Trifluoromethyl-2-hydroxypropanoic Acid: An In-depth Commentary
Historical Development
Synthetic chemistry opened countless doors for new molecules during the last few decades. Labs in Europe and North America began exploring fluorinated organic acids in the late 20th century, hunting for compounds with unique biological and chemical properties. The trifluoromethyl group, in particular, caught attention because it resists metabolic breakdown and often lends new biological activity. Researchers moved from recipes involving pyruvic acid analogues, playing with chirality, to get to what we now call (R)-2-Trifluoromethyl-2-hydroxypropanoic acid. The path included methods for controlling stereochemistry, since one mirror image can behave much differently than the other. The advances in chiral catalysis, especially using transition metals, helped make this synthesis more practical at scale. What started as an academic curiosity moved gradually into a useful building block for the pharmaceutical field.
Product Overview
This compound stands out with a structure defined by a trifluoromethyl group attached to a propanoic acid backbone. It's no simple organic acid; the CF3 group changes almost everything about its behavior in solution and in reactions. Unlike ordinary hydroxy acids, the trifluoromethyl substitution can increase acidity and shield neighboring atoms from unwanted side reactions. These features matter to chemists searching for new drug scaffolds or developing advanced catalysts. The molecule’s chirality means it comes as two possible mirror images, called enantiomers, but only the (R) form delivers certain prized properties—be it binding to enzymes or fitting precisely into complex synthetic schemes. People finding new ways to incorporate it into bioactive molecules or advanced materials face a steep learning curve but, when successful, open up space for innovation.
Physical & Chemical Properties
(R)-2-Trifluoromethyl-2-hydroxypropanoic acid usually appears as a crystalline solid, white or off-white, readily dissolves in polar solvents like methanol, ethanol, and water. The strong electron-withdrawing effect of the trifluoromethyl group bumps up its acidity compared to non-fluorinated peers, giving it a lower pKa. The hydroxy group adds extra hydrogen bonding capacity, influencing crystallization and solubility, two things that matter a lot during formulation or purification. Boiling and melting points come in a bit lower than more basic hydroxy acids, due to this altered polarity and molecular weight. Because fluorine atoms guard the molecule, (R)-2-trifluoromethyl-2-hydroxypropanoic acid shows good thermal stability, which helps during industrial processing and storage. Its chirality demands careful tracking, as racemization can erase the intended benefits of the pure (R) enantiomer.
Technical Specifications & Labeling
Most suppliers offer purity above 98%, with stereochemical composition documented by optical rotation or chiral HPLC trace. Labels indicate batch number, storage recommendations—often below room temperature, in sealed containers away from humidity—and provide regulatory compliance data. Full structural identifiers, including InChI and SMILES, make laboratory communication accurate. For research or industry use, technical datasheets cover quality analyses for each batch, laying out key figures like assay content, moisture level, and impurity profile. Material Safety Data Sheets (MSDS) spell out hazard codes, first aid protocols, and required personal protective equipment (PPE), crucial for busy labs or factories handling multiple chemicals at once. You won’t see this kind of detail unless demand from regulated industries pushes documentation standards higher, as it did in pharmaceuticals and agrochemicals.
Preparation Method
Most modern routes use an asymmetric reduction of the corresponding trifluoromethyl keto acid, employing either chiral catalysts or enzymes to set the (R) configuration. One way involves catalytic hydrogenation using rhodium or ruthenium complexes with specific ligands tuned for that crucial stereo outcome. Others steer towards biocatalysis with engineered dehydrogenases, providing a milder route that sidesteps metal residues and tough separations. The key in each method is choosing the right combination of catalyst, solvent, and temperature to favor the (R) form. Work-up steps like extraction, crystallization, and recrystallization bring the product to analytical purity, though labs keep an eye out for racemization, which sneaks in during workup if conditions drift too far from ideal. These steps demand solid technical skill, especially scaling from gram to kilogram quantities for industry use.
Chemical Reactions & Modifications
The molecule lends itself to esterification or amidation at the carboxylic acid, allowing chemists to hook it into larger scaffolds or alter solubility. The alcohol group reacts cleanly with standard protecting groups, like silyl or benzyl ethers, so users can control reactivity during lengthy synthesis campaigns. The trifluoromethyl group resists most attempts at further substitution, one reason it still interests medicinal chemists: it locks in certain biological and physicochemical traits. Under basic or acidic conditions, the molecule stands up to relatively harsh conditions, letting it serve as a platform for solid-phase synthesis or combinatorial chemistry. You can see it featured in patent filings as a building block for kinase inhibitors, enzyme modulators, or as a chiral auxiliary in asymmetric synthesis schemes.
Synonyms & Product Names
Other names stand in for (R)-2-Trifluoromethyl-2-hydroxypropanoic acid, reflecting its structure and chirality. You’ll find it under names like (R)-2-Hydroxy-2-(trifluoromethyl)propanoic acid, (R)-2-(Trifluoromethyl)lactic acid, or simply (R)-CF3-lactic acid in research catalogs. The code numbers assigned by chemical vendors sometimes hide the true identity, so users check CAS numbers for clarity—though those are often specific to the enantiomer. For custom syntheses or intermediates, it’s common to see abbreviations like (R)-TFM-LA, especially in patents and research articles.
Safety & Operational Standards
Anyone working with (R)-2-Trifluoromethyl-2-hydroxypropanoic acid reads the MSDS before starting, because fluorinated acids carry unique risks. Direct contact can irritate skin, eyes, and mucous membranes, so gloves and goggles stay on until cleanup ends. Inhalation risks are relatively low due to low volatility, but dust or fine particles must be kept out of the air. Spill protocols call for neutralization with bicarbonate and careful disposal, since treatment plants and natural environments struggle with fluorinated waste. Many labs now have custom SOPs to track inventory, restrict access to trained users, and review personal exposure limits set by organizations such as ACGIH or OSHA. Fume hoods, spark-proof refrigerators, and sealed containers turn from suggestions to requirements once scale crosses the 100-gram threshold or projects shift to GMP or GLP settings.
Application Area
This acid finds a role in pharma as a building block for new drugs, since its structural properties can boost metabolic stability and alter polarity. Researchers use it to design enzyme inhibitors or test the limits of structure-activity relationships in lead optimization. It appears in asymmetric synthesis both as a starting material for new chiral compounds and as a tool to introduce the challenging trifluoromethyl motif. Material scientists experiment with it in advanced polymers or coatings, looking for new ways to exploit the strong C-F bond and high polarity. Some agrochemical companies screen derivatives for herbicidal or fungicidal effects. Given the relentless search for new fluorinated motifs in pharmaceuticals, reagents based on the (R) acid have become almost routine in contract research labs and innovation-driven startups. Clinical chemistry teams even use it as an internal standard in certain analytical workflows, leveraging its unique mass spectrometry signature.
Research & Development
Universities and R&D departments chase better catalysts to produce this acid with higher selectivity and lower environmental impact. Chemists publish new protocols cutting out rare metals or hazardous reagents, turning to greener solvents and recyclable enzymes. Academic groups probe the biological activity of new analogues, hoping to land on novel leads for treating metabolic or CNS disorders. Often, research involves tweaking the hydroxy or carboxylic groups, testing biological uptake, or improving chemical stability. Work in drug development pipelines frequently compares this compound to its non-fluorinated cousins, seeking that edge in cell permeability, oral bioavailability, or resistance to breakdown by liver enzymes. Advanced analytical tools like NMR, X-ray crystallography, and chiral chromatography document every stage, because even minor tweaks shift properties in noticeable ways. Investment from venture funds and government grants often follows successful demonstrations of new derivatives with real-world promise.
Toxicity Research
Data continues to emerge on the long-term safety profile of (R)-2-Trifluoromethyl-2-hydroxypropanoic acid. So far, short-term animal studies show modest toxicity at high doses, mostly linked to general acidity and some organ-specific effects, like mild liver changes in rodents at repeated large exposures. The trifluoromethyl group, while common in many modern pharmaceuticals, complicates prediction of metabolic fate since fluorinated metabolites can linger longer in tissues, raising safety concerns for chronic exposure. Rigorous in vitro screens cover genotoxicity, cytotoxicity, and potential for bioaccumulation. Not all regulatory agencies have caught up to these specialty acids, even as research ramps up. Large-volume industrial users monitor worker health and environmental impact, noting that fluorinated byproducts require special attention to avoid persistence in the environment. Ongoing work to clarify breakdown routes, bioaccumulation, and eco-toxicology will hammer out best practices over the coming years.
Future Prospects
Enthusiasm for this molecule shows no signs of waning. Pharmaceutical firms continue betting on fluorinated acids as ways to jump regulatory and biological hurdles encountered with traditional building blocks. Synthesis companies invest in continuous-flow reactors and biocatalytic approaches, seeking not only efficiency but also to meet stricter environmental targets. Regulatory scrutiny supports a growing call for sustainable process design, waste treatment, and transparent supply chains. Fundamental research pushes for new analogues modifying the trifluoromethyl and hydroxy sites, aiming to coax out unexpected properties or unlock new bioactivities. The trend towards more sophisticated, functionally dense molecules draws on the chemistry pioneered for (R)-2-Trifluoromethyl-2-hydroxypropanoic acid. Demand for safer, faster, and cleaner production will keep driving search for improved catalysts, greener reagents, and alternative solvents. The next decade promises plenty of data and more than a few surprises as the applications and impacts widen across science and industry.
Straight to the Core: What Is This Molecule?
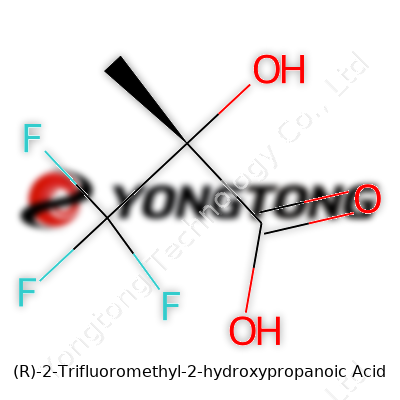
This compound, with a name that leaves many frowning, gives a glimpse into the fascinating world of fluorine chemistry. Packed neatly along its carbon backbone, (R)-2-Trifluoromethyl-2-hydroxypropanoic acid grabs attention for its unique chemical structure—one carboxylic acid group, a hydroxyl (OH) on the central carbon, and a trifluoromethyl group perched on the same carbon. The “R” means this molecule leans into right-handed chirality, an aspect that often makes or breaks biological usefulness. If you’ve ever wondered why handedness in molecules gets pharmaceutical companies worked up, this is why: the wrong ‘hand’ can turn a life-saving drug into a dud or even a danger.
Looking at the Construction
Structurally, this molecule runs like this: start with three carbons in a row. The first carbon carries a carboxylic acid group, the second carbon wears both a hydroxy (alcohol) group and a trifluoromethyl group, and the third is just a hydrogen. Those three bright fluorines bonded to a single carbon throw typical organic chemistry rules out the window, as fluorine’s stubborn pull on electrons means this molecule behaves much differently than its non-fluorinated cousins. Its chemical formula displays this bold addition: CF3–C(OH)(H)–COOH.
Fluorine’s Power
From experience in the lab, I’ve seen fluorines change the entire vibe of a molecule. It’s not a casual swap—these atoms transform boiling points, acid strengths, and sometimes the way an organism even recognizes the compound at all. Having three fluorines attached to one spot makes that end of the molecule very resistant to breaking down (resistant to metabolism), a trait that draws in drug designers hunting for stable candidates. This stability can push molecules over the finish line for pharmaceuticals that need to resist the body’s grinding enzymes. For those in public health and medicine, such chemical robustness can translate to longer-lasting drugs or even a whole new realm of therapy for stubborn diseases.
Real-World Applications and Challenges
Researchers see this structure and imagine all sorts of sharp-edged tools. The hydroxy and carboxylic acid groups open doors for linking onto larger molecular frameworks or slipping into existing biological pathways. Fluorinated compounds like this have shaped medicine, from better antidepressants to anti-inflammatories. Yet, it isn’t all sunshine and breakthrough therapies—making these tightly engineered molecules often soaks up time, energy, and money. Waste chemicals from fluorine chemistry can become persistent environmental headaches. Those same properties that make a drug last longer in your body can mean long persistence in soil and water.
Solutions begin with careful design at the drawing board. Chemists weigh both usefulness and eco-friendliness, leaning into cleaner synthesis techniques and building molecules meant to break down outside the body. For industry, routine monitoring for environmental release remains crucial. Regulators get involved, pushing safety standards, so a win in the lab doesn’t turn into a loss out in the world.
Why It Matters
The odd, powerful structure of (R)-2-Trifluoromethyl-2-hydroxypropanoic acid shines a light on the beauty—and burden—of chemical innovation. Molecules packed with function don’t come free of trouble. As society presses onwards, the challenge lies in grabbing all the benefits, minus the long-term costs. People in science, policy, and business all shoulder a piece of that puzzle, deciding if and how a compound like this improves daily life without dimming the future.
A Staple in Drug Development
Anybody working in organic chemistry or pharmaceutical labs eventually comes across (R)-2-Trifluoromethyl-2-hydroxypropanoic acid. This compound has carved out a spot in the world of drug discovery. Its chiral center and fluorinated side group make it a go-to building block for creating molecules that pharmaceuticals demand. The trifluoromethyl group, in particular, offers special qualities: it boosts the metabolic stability of many drug candidates, increases their bioavailability, and can help dial in the right balance between potency and safety. If a chemist needs to develop an antiviral, an antidiabetic agent, or even some newer types of cancer treatments, this molecule might come off the shelf more often than people realize.
Key Ingredient for Agrochemicals
Agriculture faces pushback from an endless parade of pests and blights. To deal with these, agrochemical researchers look for molecules with rugged properties, selective actions, and a low footprint on the environment. The trifluoromethyl group acts like a shield, making molecules tougher against breakdown and more effective in small amounts. That means (R)-2-Trifluoromethyl-2-hydroxypropanoic acid doesn't just contribute to more reliable crop protection; it can help cut down on runoff and unintended environmental impacts. Fluorinated compounds in agriscience lead to more efficient use of resources, which only adds to the value of compounds like this in today’s climate-pressured supply chains.
Chemical Synthesis: A Linchpin for Chiral Diversity
Lab techs and graduate students learn fast that asymmetric synthesis can get expensive and time-consuming. Chiral building blocks, like (R)-2-Trifluoromethyl-2-hydroxypropanoic acid, take some pain out of that process. Having a stable and reliable chiral center gives chemists more control over stereochemistry, which drives the function of pharmaceuticals and specialty chemicals. Getting the right “handedness” separates a life-saving drug from a dud or even a dangerous byproduct. Synthetic efficiency isn’t just a buzzword here; workforce hours and raw materials mean money. This is one of those specialty acids that can shave hundreds of hours off a complex medicinal-chemistry project.
The Environmental Challenge
The holy grail for chemists is finding materials that work well and don’t overstay their welcome in ecosystems. Fluorinated compounds sometimes raise red flags, given their durability. Companies need to rethink waste-management strategies, invest in closed-loop manufacturing, and back more research into greener alternatives. Several academic labs have started environmental-fate studies for these acids, but industry could do more to share data and push for improvement. Anyone who’s worked cleanup knows the headaches that pop up from poorly handled fluorinated waste.
Building Smarter Chemistry for the Future
Academic and industrial labs rely on tools like (R)-2-Trifluoromethyl-2-hydroxypropanoic acid to make better drugs and smarter agrochemicals. It’s about using stable, reliable building blocks, but also about facing up to the footprint left behind. Green chemistry efforts—catalyst recycling, energy-efficient routes, and rigorous oversight of emissions—shape the future of these specialty compounds. More dialogue between researchers, regulators, and communities can make the whole process more transparent and responsible. Bringing everyone to the table sets up long-term growth that serves people, not just the bottom line.
Why Purity Standards Matter
Manufacturers and researchers pay close attention to purity when handling (R)-2-Trifluoromethyl-2-hydroxypropanoic Acid. Everything from drug development to advanced materials depends on reliable chemical quality. Variations, or tiny impurities, can ruin batch consistency or trigger unpredictable reactions. Even the slightest contamination spells trouble for pharmaceutical syntheses or when scaling up procedures.
Accepted Purity Levels
Pharmaceutical and fine chemical sectors usually set the bar at 98% purity or above for this kind of compound. Researchers often push for a minimum of 99% if they plan to synthesize APIs or perform enantioselective transformations. Genuine stability and reproducibility rely on trusted numbers straight from supplier certificates of analysis, with HPLC and NMR serving as the gold standards for measurement.
Reputable suppliers post exact values. Sigma-Aldrich, for example, specifies 98% purity for their catalog offering. Other vendors, especially those catering to targeted synthesis, sometimes announce figures up to 99.5%. Purity below 98% rarely suits high-impact applications and could mean sacrificing data quality.
Impurities and Their Risks
Even minor impurities skew experimental outcomes. In small-molecule synthesis, contaminants hinder catalyst performance and add shadows to spectral readings. Asymmetric catalysis, for instance, becomes unpredictable if the acid’s optical purity drifts. That detail matters if you work with chiral drugs, where unwanted isomers or chemical leftovers end up as real liabilities.
Other hazards include leftover solvents, heavy metals, or side products from synthesis steps. The classic trifluoromethyl group draws attention from agrochemical developers and pharma teams alike because it changes a molecule's metabolic properties. Stray byproducts interfere with intended biological activities and even compromise regulatory filings.
Analytical Controls
Every well-run lab I’ve ever seen runs checks on new batches—starting with HPLC for quantification and chiral HPLC to measure enantiomeric excess. NMR spectra offer reassurance by showing real-world structures and confirming that the compound doesn’t hide unexpected peaks. FT-IR and mass spectrometry fill in any remaining blanks. If a certificate skips these basics, alarm bells start ringing.
Laboratory heads read more than just a purity number. They want to see a breakdown of impurities above 0.1%. They ask for physical data—melting points, spectra—that line up with published references. If any detail seems off, batches get set aside without hesitation.
Getting Reliable Quality
Choosing vendors with recognized accreditations pays its own dividends. Companies with ISO 9001 or cGMP certification take extra care with both documentation and analytical testing. In my own experience, supplier transparency trumps a bargain price every time—especially where regulators expect full traceability.
For those who can't afford mistakes, working with in-house analytical teams pays off. Crosschecking the supplier’s analysis keeps research on a steady path and prevents unnecessary troubleshooting. If routine analysis flags issues, reaching out for replacement stock beats fumbling through unexpected side products.
Staying Ahead of Quality Issues
Big picture, looking past the headline purity number protects results and reputations. Whether I’ve worked in an industrial lab or a university setting, the teams I trust always pay attention to precise documentation, proven analytical controls, and supplier reliability. Purity checks may look routine on paper, but they make sure bold research keeps moving forward on solid ground.
Understanding the Risks
(R)-2-Trifluoromethyl-2-hydroxypropanoic acid sounds complicated, but think of it as another specialty chemical with quirks that demand respect, not fear. I’ve seen how even seasoned lab professionals sometimes underestimate the hazards of handling acids with reactive fluorinated groups. This compound isn’t off-the-shelf vinegar; it comes with real safety stakes. Its trifluoromethyl group ranks among the most electronegative functional groups in organic chemistry. That means it can make the acid volatile and less forgiving if left exposed or cross-contaminated. For researchers or technicians, forgetting this for a second may lead to dangerous incidents.
Temperature Matters
Keeping temperature low stands out as the first real rule. I’ve worked in labs where even minor temperature deviations seeded disaster. High temperatures can trigger decomposition, releasing toxic fumes like hydrogen fluoride. Room temp storage may tempt some, especially if bench space feels scarce, but colder conditions, like those offered by a chemical refrigerator (2-8°C), keep this acid steady and tame.
Shield from Air and Water
This acid’s sensitivity extends beyond temperature: moisture and air gradually prompt the breakdown of the sample. In one of my previous projects, a colleague ignored this advice, and a batch turned unusable in weeks. Humid air can cause it to absorb water, leading to unwanted reactions and crumbling product quality. Use well-sealed containers, preferably glass with PTFE-lined caps, instead of plastic, which could leach or crack. Air-tightness makes a difference; a desiccator filled with anhydrous silica gel or molecular sieves keeps invading moisture at bay.
Avoid Cross-Contamination
People sometimes ignore the little things. Reusing spatulas or storing the acid near strong bases or oxidizers might not seem reckless at the moment, but accidents often start there. A little spill can cause fumes or more severe reactions. Assign a dedicated space in a corrosives cabinet, far from metal storage bins or caustic materials, so accidental mixing won’t become a late-night emergency call. I remember a case where improper segregation resulted in cleanup that took hours and cost the lab valuable reagents.
Label Everything, Document Every Step
Relying on memory never works out well. Proper labeling with chemical name, concentration, date received, and hazard symbols helps everyone stay safer, especially when shift changes happen or new staff join. I keep a storage logbook for every hazardous compound in the lab. It doesn’t just protect people; it helps track stability and spot problems before they spiral.
Use Protective Equipment Wisely
Nobody enjoys suiting up in goggles and gloves, especially when short on time. I learned quickly that cutting corners never pays off, especially with reactive acids. Chemical splash goggles, nitrile gloves, and a fitted lab coat cut down risks dramatically—simple steps that keep a mistake from ruining more than a day.
Disposal and Emergency Plans
Every bottle will outlive its usefulness. Keep a plan for waste collection and neutralization, and train every handler in spill response for fluorinated acids. Knowing where the nearest eyewash and safety shower are can mean the difference between a close call and a catastrophe. Across different labs, I’ve seen the best results where these points weren't just written policies but lived routines.
Summary of Key Points
- Store at 2-8°C- Protect from moisture and air using tight glass containers in a desiccator- Keep away from bases, oxidizers, and metals- Label thoroughly and log storage/activity- Always use gloves, goggles, and lab coats- Maintain clear emergency procedures and disposal plans
Why Bulk Supply Matters
Researchers and pharmaceutical companies often run up against a wall when they try to buy specialty chemicals like (R)-2-Trifluoromethyl-2-hydroxypropanoic acid in bulk. Labs on tight timelines want to scale up promising results from bench level to industrial, but they usually run into patchy supply or sky-high prices. For those unfamiliar, this compound lands on the wish lists of chemists tilting at drug discovery. A chiral building block with a unique structure, it helps chemists assemble more complicated molecules, especially when specific biological effects depend on handedness (chirality).
Real World Experience: The Supply Bottleneck
From the supplier end, it gets tricky. Companies like Sigma-Aldrich or TCI offer this acid but mostly in gram-scale bottles. Requests for kilos come hand-in-hand with sticker shock and long lead times, if the company agrees at all. That sets up a problem: if a drug candidate or an agricultural product shows promise in initial rounds, there's still a bottleneck before any real production gets rolling. A few years back while consulting for a small biotech, we hit turbulence on a similar precursor. The chemistry worked on paper, but getting even five hundred grams took a maze of emails, restrictive contracts, and custom synthesis quotes that edged north of $10,000 per kilo.
The market reflects that risk as well. Catalog prices spiral for anything labeled "enantiopure," "CF3-containing," and "hydroxy acids," and any supply disruption—be it regulatory or logistical—ripples quickly into pricing. Not many companies have the reactor volume, expertise, or regulatory compliance for scale. Production involves more than mixing a few chemicals. Raw materials, chiral resolution steps, even the solvents—every step magnifies safety, waste, and regulatory costs, and labs just don’t invest unless they see volume orders ahead.
Current Facts on Availability
Look across online chemical marketplaces and you'll spot (R)-2-Trifluoromethyl-2-hydroxypropanoic acid available for research. Alibaba and Made-in-China list suppliers who claim big numbers, but most serious buyers hesitate. Reliability, purity, and documentation remain big concerns. Western suppliers fare better on paperwork and safety data, but price and speed rarely match the promise.
Academic research and big pharma sometimes dodge the issue by developing their own synthesis route. That takes money and know-how and pulls staff away from the main project. Others press their suppliers to invest in custom manufacturing. The ability to prepay for large batches helps, but few startups have the balance sheet for this approach.
Potential Solutions
So, how do we smooth out the supply roadblocks? More open communication between buyers and producers can help establish real-world demand, convincing suppliers that investment makes sense. Companies could team up—pooling buying power, sharing regulatory costs, pressuring producers to expand capacity. Researchers with strong routes for asymmetric synthesis should consider publishing or licensing scalable variants, making the process less of a trade secret and more of a shared resource.
On a practical front, buyers should start conversations with suppliers earlier and flag intentions well ahead of needing large quantities. Pushing for clearer data sheets, traceability, and transparency on origin gives everyone more confidence in each batch. A robust, trustworthy supply chain helps not just the individual buyer but the whole R&D ecosystem, pushing innovation from the flask to the factory floor.