Pentafluorophenol: Insight into Its Past, Chemistry, and Place in Modern Science
Historical Development
Chemistry in the late nineteenth century opened doors to aromatic fluorinated compounds, but pentafluorophenol did not receive much attention until the mid-1900s. Chemists hunting for better leaving groups in peptide synthesis soon noticed the unique abilities of pentafluorophenol. The idea of using such a highly fluorinated phenol really took off as peptide science boomed. Research groups experimenting with activated esters began to see consistently high yields using the pentafluorophenyl moiety as a partner. Over the next decades, textbook syntheses anchored pentafluorophenol into everyday organic chemistry. Its efficient leaving group ability made it a staple in labs focused on proteins and pharmaceuticals. Large catalog suppliers now keep it in inventory for university groups, startup biotechs, and major pharmaceutical makers worldwide.
Product Overview
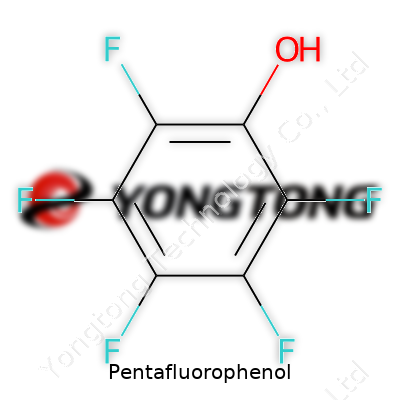
Pentafluorophenol, a fully fluorinated derivative of phenol, stands out for its electron-withdrawing properties. It delivers performance in the lab during peptide bond formation. Catalogs list it as a crystalline solid, sharply pungent to the nose, and recognized by a CAS number that rarely escapes synthetic chemists. This compound breaks the mold of ordinary phenols by trading in hydrogen bonding capacity for high reactivity and acid strength. It stands tall among activating agents, helping create esters and amides with efficiency. Its unmistakable fluorine-rich aromatic ring makes it valuable for niche applications, and the performance holds true batch after batch.
Physical and Chemical Properties
Pentafluorophenol appears as white to off-white crystalline flakes. Its melting point hovers around 84 to 86 °C, often satisfying quality control checks for purity. Unlike plain phenol, the electron cloud on the aromatic ring pulls away, helped by the five fluorine atoms clustered around the structure. This substitution pattern brings solubility in organic solvents, including ether and acetonitrile, and sharpens acidity to a pKa just below 6. Under a flask's low light, it may show slight volatility, so screw-cap bottles matter in storage. Analysis by NMR lines up five distinctive fluorine peaks, and IR shows distinct C-F stretches. Density lands around 1.6 g/cm³, solid compared to hydrocarbon-based chemicals. Reactivity with amines or activated carboxylic acids comes from the weak O–H bond, not the benzene ring, allowing it a consistent role in specialty synthesis.
Technical Specifications and Labeling
Suppliers sell pentafluorophenol under technical grades that meet or exceed 98% purity, often confirmed through HPLC or melting point checks. Commercially available packaging runs from grams to kilograms, packed with moisture-barrier liners. Labeling includes not just the CAS and chemical formula (C6HF5O), but precise hazard pictograms according to GHS. Storage guidance warns about light, humidity, and ventilation needs. Transport must follow ADR and IATA rules since it counts as a chemical reagent with special handling requirements. Final product documentation provides batch-specific analysis, including purity, melting point, and water content by Karl Fischer titration, supporting reproducible research and manufacturing.
Preparation Method
Making pentafluorophenol starts with hexafluorobenzene and cuts away a fluorine atom at the right position, typically using a nucleophilic substitution. Sodium or potassium hydroxide in polar aprotic solvents, sometimes coupled with copper catalysts, make this swap possible. The reaction calls for careful control of temperature since over-substitution or uncontrolled hydrolysis drops yield or fouls the batch. Industrial methods dial in the ratios and process time to limit side products. After the initial substitution, acidification frees up pentafluorophenol, followed by extraction into a nonpolar solvent. Filtration and rotary evaporation leaves behind the characteristic solid, which gets recrystallized for maximum purity.
Chemical Reactions and Modifications
Pentafluorophenol reacts vigorously as an acid in both electrophilic substitution and esterification. Activating carboxylic acids to their pentafluorophenyl esters immediately raises amide formation yields with less racemization. The phenol group leaves smoothly, speeding up peptide couplings sometimes bogged down by common alternatives. Nucleophilic aromatic substitution on pentafluorophenol provides an on-ramp for novel functionalizations. Silylation or alkylation of the oxygen allows for unique protecting group chemistry, and the aromatic ring itself serves as a platform for further halogen exchange or cross-coupling. Under harsh conditions, the ring resists degradation, which is why it anchors various tailored ligand systems for catalysis and sensor design.
Synonyms and Product Names
Pentafluorophenol also goes by several aliases in academic and commercial circles. Chemists sometimes refer to it as PFP or 2,3,4,5,6-pentafluorophenol. Its systematic name reflects its five fluorine substitutions. Certain suppliers label it simply as perfluorophenol, though technically this confuses different fluorine counts. In synthetic schemes, researchers often abbreviate pentafluorophenyl (Pfp) when discussing esters or intermediates, but for regulatory filings, the precise chemical name and structure must appear.
Safety and Operational Standards
Few synthetic compounds command the same level of respect in handling as pentafluorophenol. Its high acidity and strong scent can irritate mucous membranes fast. Users must work it under a fume hood, and nitrile gloves protect against accidental skin contact. Eye protection ranks as non-negotiable, with splash risks present during weighing or transfer. Spills require absorbent materials and prompt disposal — this compound does not belong in sinks or landfill. Lab safety data sheets classify it as acutely toxic by ingestion and warn employees about chronic risks. Emergency eyewash stations and showers cannot be skipped. Despite these risks, careful labeling and training mean research labs rarely see serious accidents with pentafluorophenol.
Application Area
Pentafluorophenol shaped the story of peptide synthesis, letting chemists accelerate the coupling of even hindered or sensitive amino acids. The pharmaceutical industry relies on pentafluorophenyl esters to cut waste and boost productive yields for preclinical drug candidates. Biochemistry teachers lean on pentafluorophenol’s efficiency in classroom demonstrations of esterification and amidation, bringing real-world tools into the curriculum. Material science takes up pentafluorophenol for hydrophobic coatings and as a building block for specialty polymers or surface modifiers. Chemists running solid-phase synthesis use it to cap reactive groups or modify surfaces for diagnostics. Catalysis research and chiral ligand development turn to the characteristic electron-poor aromatic ring to craft bespoke molecules or sensors.
Research and Development
Active research tracks new ways to use pentafluorophenol both as a reagent and a scaffold for next-generation materials. Environmental scientists probe its resistance to biodegradation, feeding into safety standards for downstream processing. Industrial process engineers streamline the scale-up of pentafluorophenol production, seeking greener solvents and lower energy demands. Teams focused on drug discovery explore how the pentafluorophenyl group impacts pharmacokinetics and target binding. Analytical chemists develop new NMR, GC-MS, and LC-MS methods for detecting trace pentafluorophenol in environmental and process samples. As more synthetic platforms mature, researchers test out modified pentafluorophenol derivatives in advanced organocatalysis or imaging probes.
Toxicity Research
Pentafluorophenol does not give up its secrets easily when it comes to biological effects. Toxicological studies report acute oral and inhalation toxicity in mammals, with irritation seen at low concentrations. Chronic exposure risks have not been fully resolved, but the fluorinated aromatic structure raises concerns about persistence and bioaccumulation. Routine animal testing highlights organ-specific impacts in the liver and blood after sustained dosing. Waste handling and community discharge must comply with stringent local and international environmental standards. Workers in chemical manufacturing plants face mandatory personal protective equipment and regular health monitoring. Recent studies aim to track its environmental fate and propose best-in-class remediation processes that minimize ecological impact.
Future Prospects
As green chemistry pushes innovation, pentafluorophenol’s traditional strengths in activating esters and stable leaving groups get challenged by biodegradable alternatives. Still, demand for high-efficiency peptide synthesis and specialty functional materials keeps academic and industrial interest alive. Next-generation research tries to tune the reactivity of pentafluorophenol derivatives, finding gentler and more sustainable synthetic partners. Industry looks at improved containment, recycling, and recovery to close the loop on fluorinated compound use. Regulatory agencies keep gathering data to guide exposure limits and environmental discharge, with trends pointing towards tighter restrictions unless safer alternatives emerge. For now, pentafluorophenol keeps its niche as a high-performance specialty reagent in advanced chemistry and materials applications.
Why Pentafluorophenol Catches the Attention of Scientists
Pentafluorophenol rarely pops up in everyday conversation. In research labs, though, it draws a crowd of chemists who count on it for more than its tricky pronunciation. I remember encountering it during my graduate days, cringing at the thought of storing something with a reputation for volatility and a harsh smell. Yet after a few months in the lab, its usefulness clicked—a small bottle could make or break an experiment in the world of peptide synthesis.
The Real Use: Making Bonds Stick
Researchers lean on pentafluorophenol to help make strong connections between molecules. It works as an activating agent, giving a boost in making special chemicals called esters. Pentafluorophenol-based esters step up in the coupling of amino acids, which matters a lot when building peptides and proteins by hand—a foundation for drug discovery and biotechnology. Peptides aren’t just academic; they lead to insulin, cancer treatments, and even some of the more advanced COVID-19 therapies.
In the quest to rapidly and reliably stick amino acids together, pentafluorophenol stands out for making “active esters” that speed up the process and leave less mess behind than similar chemicals from older generations. These reactions save money and time, letting labs pump out cleaner products in higher yields. I saw firsthand how colleagues wasted days chasing after reactions with cheaper, less effective chemicals, only to switch to pentafluorophenol and finally get the results they were after.
The Balance of Power: Safety, Environment, and Ethics
Having a tool like pentafluorophenol doesn’t mean throwing caution to the wind. Its ability to enhance chemical reactions comes with a sharp edge. The stuff is toxic and has a way of lingering in the environment. Disposable gloves won’t protect skin for long, and spills require real attention during clean-up, standing out even in crowded storerooms.
Laboratory safety officers keep eyes peeled for careless use, because long-term exposure can harm lungs and eyes. Environmental agencies in Europe and the U.S. track its disposal with stricter rules each year. Researchers must invest in training and proper waste handling, even when budgets feel pinched. Some pharmaceutical companies have explored “greener” alternatives, pushing the market to search for activating agents that break down naturally without polluting soil and water. I’ve seen a few startups try to develop these solutions, but so far, pentafluorophenol holds its place because the alternatives don’t always match its performance.
Looking Ahead: Smarter, Safer Chemistry
The demand for blockbusters in science—better drugs, novel materials, biotechnologies—drives people to substances like pentafluorophenol. At the same time, sustainability isn’t a buzzword in scientific circles anymore; it’s a basic expectation. Some academics have called for global registries tracking chemical footprints, similar to how hospitals trace antibiotics. A colleague shared that his lab started labeling reagents for their “green score,” encouraging young chemists to think twice before reaching for the usual suspects.
Pentafluorophenol’s strengths give it a secure role in modern organic chemistry, but every scientist using it is always calculating the risks, cost, and benefit—both in the lab and well beyond. The next chapter may involve smarter design, safer alternatives, or even brand-new approaches that cut out risky chemicals altogether. Lessons from pentafluorophenol keep echoing in research meetings, driving home the value of experienced choices and real-world safety.
Understanding the Stuff
Pentafluorophenol doesn’t get much attention outside the lab, but it’s worth looking at because it keeps showing up in chemical synthesis and research. This compound isn’t something people stumble upon during a walk outside. Chemists use it to activate carboxylic acids and for making things like esters and amides — usual building blocks for drugs and advanced materials. The thing looks pretty harmless in a bottle, a white crystalline solid that can trick people into thinking it’s just another boring reagent. The reality is a bit different.
Breathe It, Touch It: What Happens
Every safety sheet I’ve read about pentafluorophenol reads like a warning sign. The main issue is exposure. Just being near this chemical in its pure form calls for caution. The stuff gives off fumes that stick to your nose and throat. A splash can sting the eyes and skin. Some folks compare the smell to sour, biting plastic. If you breathe the dust, the lungs tend to complain, which could mean coughing, tight chest, or irritation you can’t wave away with a few sips of water.
Long-term studies on people haven’t been done, probably because nobody hangs out with pentafluorophenol for fun. Lab rats and test tubes tell enough of the story: contact damages tissues, and eating it is a worse idea. In the worst cases, severe exposure causes burns or could even knock out your organs, though most scientists would never come close to this scenario because of the strict lab controls.
Toxicity: What the Science Says
Pentafluorophenol carries risks similar to other highly fluorinated aromatic compounds. Its structure gives it staying power in the body and environment, not unlike some of the “forever chemicals” making news lately. Some toxicity studies show harmful effects on cells and living creatures, with impacts on liver and kidney tissues. Many chemicals in this family build up over time, putting extra stress on organs even at low levels of exposure.
To make matters trickier, it’s not yet clear exactly how much is too much, especially since most encounters happen in the lab. This uncertainty keeps chemists on their toes. It’s not as acutely toxic as old-school pesticides or outright poisons, but it’s no sugar pill either. “Harmful” and “irritant” show up time and again in documents from health and safety regulators in the US, Europe, and Japan.
Direct Experience in the Lab
Nobody who works with pentafluorophenol treats it lightly. Glove changes, face protection, fume hoods, emergency showers — they all get used far more often in labs where this reagent pops up. Once, during a graduate project, I watched a single drop corrode a plastic glove, and after that, our group treated every bottle with extra care. We kept it double-sealed and logged every use. Open-air handling meant full-face shields and backup ventilation. Every waste container got labeled in angry red so custodial staff wouldn’t get a nasty surprise. Even the most basic training included pentafluorophenol as a “respect this chemical” marker.
Solutions: Working with Respect
Most problems linked to pentafluorophenol come from taking shortcuts or missing information. More up-to-date safety training helps keep mistakes from happening. Chemical fume hoods, eye washes, and good gloves save hands and lungs from unnecessary pain. Waste gets treated as hazardous, not normal trash. Some chemists have tried finding safer alternatives, but for now, the most reliable move is to follow protocols and flag every risk. Using less of the chemical and swapping in greener choices if possible would make labs safer. People working with this stuff owe it to themselves and their colleagues to share warning signs and best practices, not just rely on books or regulations.
The Nature of Pentafluorophenol
Pentafluorophenol shows up in chemistry labs often enough, especially in peptide coupling and other organic synthesis work. Its sharp, distinctive odor makes clear that it demands respect. Anyone who has worked with this compound knows its volatility and potential health hazards just by that first breath—eye and airway irritation follows quickly if you’re careless.
Why Careful Storage Matters
A simple container on the wrong shelf spells trouble, and plenty of researchers have experienced the hassle of accidental exposure or spoiled product. Pentafluorophenol reacts quickly with moisture and carries toxic risks, meaning proper storage keeps both you and your research safe. I remember a colleague who ignored the label’s warnings and stored a bottle near open acids. The melt in the glass stopper made a sticky, noxious mess no one wanted to clean up.
Core Storage Practices
- Cool, Well-Ventilated Area: Heat speeds up pentafluorophenol’s volatility and decomposition. Keeping it in a cool space lowers vapor risks and avoids pressure buildup in the bottle. In my time, a simple mistake with temperature control led to pressure leaking the cap loose and filling the cabinet with fumes. Nobody wants to repeat that.
- Keep the Container Tightly Sealed: This may sound obvious, but a loose lid or a recycled container without proper lining quickly spoils the purity. The fumes escape and the compound absorbs moisture from the air. Once, a junior team member thought parafilm alone would work—by morning, both air and product were contaminated.
- Store Away from Acids and Bases: Many organic chemists store reagents together, but pentafluorophenol needs isolation. Mixing it nearby incompatible chemicals increases the chance of dangerous reactions. Segregate by compatibility. I’ve seen old vials corroded on one side because they sat near nitric acid—luckily caught before anything erupted.
- Use Non-Metal Containers: Glass or certified non-reactive plastics prevent unwanted interactions. Metal caps or shelving give a path for accidental reactions over months of storage.
Labeling and Safety
A clear, durable label makes life easier. People forget or containers get misplaced, so good identification outlasts interns and students coming and going with each semester. Mark the date received and every time you open it. This helps anyone tracking decomposed bottles or planning for disposal.
Good labs also keep safety sheets—SDS, procedures, emergency numbers—close to storage areas. I’ve learned some of the best advice from a well-maintained binder. New team members pick up safety knowledge by osmosis once materials are easy to find.
Disposal and Handling Spills
No one likes cleaning up after spills, but quick action minimizes harm. Absorbent pads, gloves, fume hoods, and a working knowledge of your lab’s protocol matter. Pentafluorophenol clings to surfaces and lingers in the air, so ventilation comes first. For waste, sealed containers and labeled hazardous waste bins keep environmental risks down. Disposal companies prefer clear labeling and safe containment; mishandling can lead to expensive cleanup bills.
Real-World Solutions for Safe Storage
Labs old and new may neglect clear organization. Assigning responsibility goes a long way. An experienced researcher or technician regularly checking on chemical storage prevents the kind of small issues that grow into bigger accidents. I’ve seen labs adopt checklists for storage checks every month; compliance and safety both go up. Training pays dividends, especially for students. Once everyone knows the risks, peer pressure reinforces habits, and the storage question becomes second nature.
Unlocking the Formula: C6HF5O
Pentafluorophenol, with the tidy formula C6HF5O, shows how powerful a single substitution on the classic benzene ring can be. Swapping out five hydrogens for fluorine atoms, chemists end up with a compound that’s much more than a member of the phenol family. This change doesn’t just tweak the molecule; it gives pentafluorophenol qualities that keep it in high demand among researchers.
What Makes Fluorine Substitution Stand Out?
Fluorine isn’t your everyday atom. It’s highly electronegative—which means it pulls electrons toward itself—and it holds up under harsh conditions. I remember my own surprise in the lab the first time I worked with a fluorinated compound. The resistance to breakdown and the way these compounds shrugged off strong acids and oxidizers stood out right away. Pentafluorophenol takes these properties and runs further. Thanks to those five fluorines, this molecule becomes more acidic compared to the basic phenol, which isn’t something you see every day in a simple aromatic compound.
Why does this matter? A more acidic molecule acts as a stronger leaving group, making reactions faster and more efficient. In practical terms, chemists tap into pentafluorophenol when they want to link molecules together—like building complex peptides for medicine or probing biological systems. It’s not just about mixing chemicals; it’s about saving time, reducing side reactions, and hitting high yields with less purification needed later.
The Role in Peptide Synthesis
If you browse the literature, pentafluorophenol pops up often with peptide coupling agents such as DCC or EDC. The goal: activate a carboxyl group so it can hook up with an amine to form an amide bond. This process may sound routine, but in reality, every step can present challenges, especially with tricky amino acids. Pentafluorophenol’s unique behavior stems from the pull of those fluorines, which makes the carboxyl-activated intermediate easier to attack. Anyone who has searched for predictable yields knows how important this boost can be. Peptide synthesis forms the backbone of drug discovery and diagnostics. Tight budgets and deadlines make it essential to use reagents that work reliably. Pentafluorophenol’s presence in the lab speaks to its consistent performance under pressure.
Environmental and Safety Considerations
There’s another side to the pentafluorophenol story. With fluorinated chemicals, disposal and handling rules get strict. The stability that makes these molecules so attractive in the lab means they can linger in the environment. It isn’t just a laboratory concern—over time, these compounds can move through water systems and bioaccumulate. That’s why chemists pay careful attention to waste protocols. Proper containment, recovery, and adherence to regulations help offset the risks and keep their benefits from turning into liabilities.
Pathways Forward
Forward-thinking labs seek greener synthesis routes. Since pentafluorophenol remains valuable, researchers experiment with recycling strategies and alternative coupling agents. Academic journals highlight both its efficiency and environmental impact. I’ve seen workspaces adopt strict collection procedures, and companies invest in safer analogs. This approach doesn’t throw out the old trusted tools but considers new directions for sustainable research.
Understanding What’s on the Bench
Pentafluorophenol stands out as a useful chemical in the synthesis world but also brings its share of safety concerns. It’s easy to forget what makes a compound so nifty for building complex molecules can also make it unpredictable in your beaker. This chemical’s reactivity doesn’t just make it a staple for esterifications; it makes even experienced chemists reach for gloves and splash goggles as a matter of instinct rather than rule-following.
Real Hazards, Real Consequences
Fluorinated aromatic compounds don’t play nice with skin or lungs. Pentafluorophenol gives you a sharp, stinging odor warning, but you’d rather not get close enough to notice. The liquid and the dust both irritate the eyes and respiratory tract. There’s data pointing to toxic effects in animal studies, with enough on record to prompt special caution in human handling. No one collecting their own horror stories about chemical burns or coughing fits wants to repeat the lesson. The point hits home: one slip or spill lingers as more than just an inconvenience.
Personal Protective Equipment is the Starting Point
No matter how routine the procedure, nitrile gloves and a lab coat become armor, not afterthoughts. Face shields and chemical splash goggles serve a practical role. A fume hood isn’t an overreaction—it’s just where pentafluorophenol belongs. Labs with a culture of safety keep practices visible. Anytime the vial opens, everyone nearby registers what’s happening.
Storage Habits That Matter
This compound stays in tightly sealed bottles, usually glass, away from acids, bases, and sources of heat. Most labs mark the container and track its location to keep accountability clear. Refrigeration reduces volatility and keeps it stable over time. The main motivation for care stems from a desire to keep emergencies from happening, rather than fixing trouble after it starts.
Cleanliness and Spills: Everyone Owns the Mess
Cleaning glassware after use feels like a chore until you share a bench with someone careless. Rinsing with solvent and double-checking for residues turns into mutual respect. Spill kits get stocked because someone always knocks over something eventually. Absorbent pads and neutralizing agents handle accidents on the spot. Every chemist knows the value of a clean, organized workspace because it means fewer surprises in the next experiment.
Waste Handling and Environmental Responsibility
Disposing of pentafluorophenol follows rules, never shortcuts. The chemical shows persistence in the environment. Collected waste goes into labeled bottles, not down the drain. Licensed hazardous waste disposal companies handle the final step. Labs following regulations don’t just avoid fines—they prevent a bigger problem downstream.
Why Experience Adds Up
Trust builds among colleagues who share safety habits. Lab veterans remember the small burns and near-misses, and make sure students pay attention. Supervisors who model good handling practices earn respect. Training newcomers includes mentioning real risks as much as reaction yields. In my own experience, the best advice comes from stories traded over a coffee break about what went wrong and how to avoid it next time. Leveraging lived examples gives everyone an incentive to take pentafluorophenol seriously and keep the bench—and everyone behind it—safe.