Para-Toluene Sulfonic Acid: A Commentary
Historical Development
Para-Toluene Sulfonic Acid (PTSA) has a story rooted in the rapid industrial advances of the late nineteenth and early twentieth centuries. The growth in demand for synthetic dyes and advanced resins gave rise to a whole family of sulfonic acids. PTSA found early utility as dye intermediates and grew into a staple for chemical manufacturing thanks to straightforward preparation from toluene and sulfuric acid. Chemists chased better acids for organic synthesis for years, and PTSA landed in the sweet spot—strong enough to drive reactions forward but easier to control and less volatile than mineral acids like sulfuric. I’ve seen this evident in older industrial texts and lab archives, where batches of sulfonic acids were the stepping stones to much of modern polymer chemistry and pharmaceuticals. Hard to ignore the legacy; it’s one that shows how engineers and bench chemists, through trial and repeated error, honed the process and put their faith in simplicity and reproducible results.
Product Overview
PTSA appears as white or colorless crystals, emitting a distinctly sharp, sour scent. Chemists and plant workers respect it for its reliability. It serves as much more than a catalyst—it’s an anchoring acid in a range of industries. My years handling PTSA emphasized its role as both a cleaning agent and a reagent. Both roles demand stability and a strong, non-smoky acidity that doesn’t corrode metal equipment as harshly as others might. PTSA goes by the moniker p-Toluenesulfonic acid, or just tosylic acid, showing up in shipping manifests and laboratory catalogs worldwide.
Physical & Chemical Properties
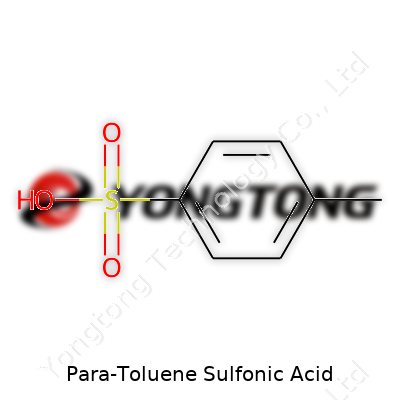
PTSA holds a melting point near 103°C and dissolves freely in both water and alcohol, making it adaptable in laboratory and plant settings. Density checks out at around 1.24 g/cm³. It stands as a solid at room temperature, which means storage and weighing stay simple. Chemists trust its acidity (pKa ~ -2.8), placing it among the stronger organic acids but less risky than concentrated mineral acids that fume and burn on contact. PTSA’s molecular structure features a benzene ring with a methyl group and a sulfonic acid group, locking in both hydrophobic and hydrophilic properties.
Technical Specifications & Labeling
Suppliers usually market PTSA in two forms—monohydrate and anhydrous. As a buyer or handler, it’s crucial to distinguish between the two; water content shapes both acidity and applications. Labels should display concentration, batch number, and hazard symbols, following guidelines laid down by regulatory authorities. Clear identification on drums and small-lab containers isn’t optional. My experience has taught me that even minor lapses in marking containers can trigger costly delays and safety reviews, underscoring the value of precise specification.
Preparation Method
Much of the PTSA in use today springs from the reaction of toluene with concentrated sulfuric acid, producing a mixture of sulfonic acid isomers. Fractional crystallization sorts out the para-isomer, leaving the ortho form behind. This separation relies on crystallization techniques, not solvents or columns, which cuts down on cost and complexity for industrial operators. Plant engineers favor continuous procedures to scale up production, and throughput remains high with careful temperature management. Having worked with both batch and continuous systems, there’s no comparison—continuous flow cuts down on inconsistencies and puts control in the hands of operators.
Chemical Reactions & Modifications
PTSA acts as a strong acid catalyst; esterifications and polymerizations depend on its presence to run efficiently. Many synthetic schemes lean on PTSA to activate carbonyl compounds or facilitate dehydration reactions. Chemists also use it to create sulfonate esters, like tosylates, which serve as great leaving groups in organic synthesis. I’ve prepared dozens of tosylate derivatives under mild conditions, avoiding harsher alternatives that risk side reactions or hard-to-remove byproducts. PTSA’s combination of reactivity and compatibility keeps it on the shopping list for custom syntheses, both academic and industrial.
Synonyms & Product Names
You’ll spot PTSA labeled as p-Toluenesulfonic acid, para-Toluenesulfonic acid, and 4-Toluenesulfonic acid on chemical invoices and research articles. The abbreviation TsOH shows up just as often in academic papers—less cumbersome, but just as accurate for those familiar with the field. Commercial grades may specify “monohydrate” or “anhydrous” depending on water content. It pays to double-check paperwork for these details, as I’ve learned from seeing failed reactions caused by an unexpected water load.
Safety & Operational Standards
PTSA’s strength as an acid brings risk as well as utility. Skin contact burns and eye injury rank high among workplace accidents. Proper gloves, goggles, and fume extraction are non-negotiable when working with the powdered or crystalline acid. PTSA solutions, especially in alcohol, generate heat when mixed with water, so slow addition under stirring stays good practice. Disposal falls under local environmental legislation; wastewater with PTSA must be neutralized before reaching drains. I always train new staff to err on the side of caution—most incidents stem from rushing or improvising with unfamiliar materials.
Application Area
PTSA turns up in a remarkable range of settings, from paint factories to pharmaceutical plants. Esterification reactions in polyester resin production, surfactant manufacturing, and batch syntheses of fine chemicals all draw on its catalytic properties. In labs, it cleans glassware quicker than nearly any other acid, stripping tough organic residues in minutes. As a solid acid catalyst, PTSA outperforms mineral acids in selectivity, limiting over-reaction and reducing unwanted byproducts. I’ve watched industrial customers replace older acid catalysts in resin production lines, earning higher yields and lower corrosion rates—proof positive that PTSA carves out a unique niche.
Research & Development
Research groups keep an eye on PTSA as both a reagent and a target for modifications. Novel catalyst systems spring from PTSA derivatives, such as immobilized acids on silica or polymers. These hybrid catalysts enable easier separation and reuse, which aligns with green chemistry principles. Chemists have also tested PTSA in water-sensitive reactions, pursuing methods that dial up product purity and minimize waste. In my own projects, I’ve found value in these developments, especially where regulatory targets or sustainability goals demand more selective and less hazardous methods.
Toxicity Research
PTSA poses a low risk of chronic toxicity to humans compared with many other industrial acids, but acute exposure burns skin and damages mucous membranes. Animal studies show irritation following ingestion or inhalation, while environmental studies flag risk to aquatic life if PTSA-tainted runoff enters waterways. Standard practice in industry keeps air concentrations low and enforces rigid spill response. From my perspective, the blend of acute hazard and low chronic risk justifies current control measures—regular training and reinforced personal protective gear stop most problems before they start.
Future Prospects
More industries look to PTSA for new polymer syntheses, greener routes to pharmaceuticals, and more efficient fuel additives. Catalysis research points toward modified PTSA derivatives for specialty applications. Environmental regulation continues to push users toward catalysts and acids that can be recovered and recycled, making immobilized PTSA an area of both academic and commercial growth. Researchers concentrate on cutting side products and improving selectivity, so customers can demand both quality and environmental responsibility. My experience says the story remains unfinished—each year brings new reactions and improved formulations, fueled by challenges in production scale and sustainability.
Behind the Chemistry: What Sets This Acid Apart
Pouring through the shelves in any chemical supply warehouse, certain bottles catch the eye. Para-toluene sulfonic acid, or PTSA for short, isn’t flashy—but it’s a quiet workhorse. My time helping out at my uncle’s small resin production facility hammered home the kind of roles this compound fills. Spilled a little once and watched resin polymerization grind to a standstill—lesson learned: PTSA doesn’t just help reactions along, it sometimes keeps things from falling apart.
Boosting Resin and Adhesive Production
PTSA stands out in the resin industry. Companies building adhesives or coatings rely on strong, predictable chemical reactions. This acid acts as a catalyst in making phenolic resins and amino resins. Instead of using something more volatile or dangerous, they pick PTSA to push methylation and condensation reactions safely and consistently. In furniture and plywood, the reliability of the bonds owes a lot to this chemical. A poorly cured board leads to splinters and wasted resources, so companies trust what works batch after batch.
Improving Everyday Cleaners
In my house growing up, a sticky label meant soaking in solvent or picking for hours, but liquid cleaners today tackle messes much better. PTSA plays a big role in surfactant and detergent production. It creates the sulfonation reactions that give detergents their punch against grease and grime. Hand dishwashing liquids and all-purpose cleaners work smoothly because PTSA helps break down fats at a molecular level. Lab tests I read as a student showed that no matter how fancy the brand, the same basic chemistry drives the cleaning power.
Pharmaceuticals: Precision Work Demands the Right Acid
Looking deeper into pharma, the details matter even more. PTSA makes life easier during peptide synthesis and production of certain medications. It’s a solid choice for acid-catalyzed transformations, including esterification and protecting group removal. Formulating medicines requires batch-to-batch consistency, which PTSA delivers. Efficiency matters: pharma builds on reactions they can trust, and this sulfonic acid gives them room to fine-tune the production line, cutting down on waste and unpredictable side products.
Supporting Dyes and Pigment Manufacturing
Driving by textile mills, the river sometimes ran blue or green—this throws back to the important part PTSA plays in dye chemistry. Creating bright, lasting dyes needs steady acid catalysis, which this compound brings to the table. Color vibrancy in clothing, especially when you want that red shirt to stay red through twenty washes, gets a boost from the reactions it energizes. PTSA’s strong but steady approach gives pigment makers another tool, right beside the ovens and vats, to get the shade and stability customers expect.
Safety and Sustainability
Every chemical comes with baggage. PTSA is strong—touch or inhale, and problems follow. Overexposure in production settings leads to respiratory and skin issues, so I saw first-hand how important protective gear and proper ventilation remain. Companies moving toward greener production sometimes seek alternatives, but the performance-per-kilogram balance still pushes them back to PTSA. Safe storage, carefully measured use, and spill response remain constant talking points for anyone handling it.
Pushing Forward: Smarter Application Means Less Waste
Industries always hunt for ways to use less, waste less, pollute less. Teams continue to tweak how and when they add PTSA, especially as demands grow for cleaner processes. Smart dosing and closed-system handling cut down on accidents and runoff. Acid recovery technology, next-gen formulations, and diligent training all chip away at the risks—but the chemical’s place in labs and plants remains hard to replace for now.
PTSA leaves fingerprints across products and industries, linking the old with new ways of thinking. It keeps chemistry grounded and products going strong, with stories behind every batch produced safely and efficiently.
Getting to Know Para-Toluene Sulfonic Acid
Para-Toluene Sulfonic Acid, or PTSA, shows up in a lot of chemical processes, from making pharmaceuticals to manufacturing dyes and resins. Its strong acidic nature makes it a popular choice in labs and factories. That said, anyone working with PTSA learns to treat it with respect. The risks don’t always show up right away, but ignoring precautions can catch up with you.
Direct Contact and Exposure
Guy in my college chem lab always said, “A little caution goes a long way.” PTSA as a dry powder or a concentrated liquid is tough on human skin and eyes. Just a splash can cause a burning sensation, redness, and, if you’re unlucky, a nasty chemical burn. Breathing the dust or fumes feels like sniffing vinegar laced with pepper—harsh and immediate. Coughing, sore throat, and in bad cases, trouble breathing pop up as common complaints.
The Material Safety Data Sheet (MSDS) for PTSA spells out those dangers clearly. It doesn’t cause cancer as far as research shows, but it’s corrosive enough to damage tissue on contact. Folks with asthma or other respiratory issues feel the effects much sooner. Once that fine dust goes airborne, it lingers, settling on benches and clothing, making clean-up a priority.
Safe Handling and Prevention Matter
In chemical plants and school labs, safety talks about PTSA get more attention than most. Gloves, goggles, and splash-resistant coats aren’t just for show. Experienced workers often notice a tingling in the nose if fumes rise too high; well-ventilated workstations help a lot. Emergency washes and showers offer some comfort, but they’re not a substitute for being careful in the first place.
The acid stays stable under typical storage, but if mixed with strong oxidizers or bases, it reacts fast—sometimes violently. In my last job, a rushed cleanup led to a minor spill. Even without injuries, downtime and paperwork followed, so training and drills paid off. Factories that keep exposure levels within guidelines from OSHA and local authorities see fewer incidents. Regular monitoring and up-to-date safety gear feel like overkill until you face a spill for real.
Beyond the Workplace
PTSA rarely finds its way into household products. Because most folks never handle it directly, big risks outside the industrial world stay low. If it does leak out, environmental rules require fast containment to keep it away from water supplies. It dissolves easily, so local streams or rivers would carry it far if left unchecked. Most environmental agencies push for spill kits and clear reporting procedures, and for good reason.
The Importance of Hitting the Basics
Experience tells me that respecting chemicals from the start beats dealing with accidents after the fact. Para-Toluene Sulfonic Acid isn’t as famous as some other hazardous substances, but it earns its stripes among chemists for being aggressive on contact. Protecting health means more than following the rules; it means knowing how much trouble a clear, sharp-smelling liquid can cause if the basics get skipped. Qualified training, working hoods, and good habits stand between a regular day and a trip to the ER. Facts and firsthand stories both remind us—PTSA deserves caution in every setting.
Understanding the Risks Before Storage
Para-Toluene Sulfonic Acid turns up in many labs and factories because it speeds up chemical reactions, especially for making pharmaceuticals and resins. You might recognize it as a white crystalline solid or a strong liquid solution, both of which can cause real trouble if not kept properly. A lesson learned the hard way: improper storage invites corrosion, spills, and health problems for anyone who goes near it without the right protection.
Key Storage Considerations
Ignore the temptation to treat this compound like any other chemical. It eats through metal shelves, attacks exposed skin, and reacts strongly with water. I remember a facility using rusty, thin-walled steel drums. Within months, everyone was talking about mystery leaks. Turns out, a chemical like this belongs in tightly sealed plastic containers—think high-density polyethylene or Teflon, not old metal barrels.
Temperature control stands out. You won’t want heat or sunlight beating down on the containers. Heat speeds up decomposition, which could release hazardous fumes or ruin the acid’s effectiveness. At a plant I visited, drums sat near a window. By midsummer, the contents had turned brown and gave off a biting smell; the batch had to be scrapped. Indoor, ventilated rooms with steady, cool temperatures offer real peace of mind.
Humidity poses its own challenge. This acid pulls moisture from the air. Its label says “hydroscopic,” which in simple terms means it loves to absorb water. A careless lid or even a short exposure during transfer leaves a gooey mess—and the risk of a reaction. Secure those lids after every use. Throw in some silica gel packs for extra safety if the air’s muggy.
Safe Handling and Access
Para-Toluene Sulfonic Acid forms toxic fumes. Inhaling even a small amount stings the lungs and can cause lasting harm. Anyone working nearby should gear up with nitrile gloves and goggles. Ventilation matters. Chemical storage rooms need fans or hoods to keep fumes from collecting.
Accidents happen. In one situation, a bottle slipped, spilling acid on the floor and splashing a worker’s boot. Rubber gloves and quick action (lots of water, plenty of baking soda) saved the day, but only because the right supplies stood close by. A spill kit with soda ash or sodium bicarbonate can neutralize the acid fast, making cleanup safer and easier.
Labeling and Inventory Go Hand in Hand
Clear, resistant labels on every container make a difference, especially in a crowded storage room. Imagine somebody reaching for the wrong bottle without knowing it—then mixing it with the wrong chemical. Disaster follows confusion. Keeping an updated log of inventory, storage dates, and container condition prevents surprises.
Solutions That Actually Work
Training every staff member goes a long way. You can have the toughest container and the best shelves, but without people who know what they’re handling, accidents happen. I’ve seen facilities shift from yearly training to quarterly sessions, and reports of spills dropped by half.
Regular inspections make sense. Cracks in a cap or a weakened area on a drum only grow worse over time. Scheduling weekly visual checks, alongside routine inventory, turns up small problems before they grow into emergencies.
Para-Toluene Sulfonic Acid does valuable work in the right industries, but it demands respect and smart storage. A cautious approach keeps staff safe, protects the product, and helps limit avoidable incidents.
Lessons from the Floor: Why Spills Deserve Respect
Spilling para-toluene sulfonic acid does more than make a mess—this isn’t just a simple clean-up like a dropped soda can. Touching this acid means risking burns or digestive tract injuries if swallowed, and breathing in its fumes doesn’t help anyone’s lungs either. In some labs, I’ve seen people skip the gloves or eye shield guessing they’re saving time. In truth, that shortcut only stretches a spill into a story nobody wants to tell.
Paying Attention Saves Trouble
Stop the spill at its source. If a drum leaks or a beaker tips, the flow continues until you intervene. Block the route with absorbent materials—preferably pads or commercial spill pillows designed for acids, never random rags. Keep the acid contained before it finds a drain or a crack in the floor. Fast action makes the rest of the job easier.
I’ve learned the hard way that guessing with protective equipment doesn’t end well. Suiting up sounds dramatic, but gloves, goggles, and chemical aprons give small mistakes much less impact. Standard latex gloves might melt, so thicker nitrile or neoprene gloves handle the liquid better. Goggles do more than clear your conscience—they stop surprise splashes in their tracks. Face shields for large spills aren’t an overreaction either.
Neutralize with Caution
Someone always jokes about just pouring water on acid, but that’s an invite for trouble. Instead, sprinkle a solid, weak base like sodium bicarbonate (baking soda) or sodium carbonate slowly on the edges of the spill. Let the reaction do its work in manageable amounts. Expect fizzing and maybe some heat. Rushing this step can splash acid wider than the original spill area. Move slow, watch for complete bubbling, and add more as needed until the liquid stops responding. Only then use wet rags or a mop to pick up the neutralized waste.
Bag It, Tag It, Don’t Just Toss It
Lab trash cans or standard dumpsters won’t cut it for this kind of waste. Neutralized residue and dirty pads belong in chemical waste containers with clear hazard labels. Mixing neutralized acid with trash ignores rules and risks fines from regulatory agencies. Many times, I’ve seen the difference between a smooth investigation and a shutdown come down to one label. Regulatory inspectors care less about good intentions and more about proof.
Ventilation and People Matter
Spilled para-toluene sulfonic acid stings the nose, but its vapors don’t get talked about enough. Open up windows if possible, turn on local exhaust fans, and steer people out of the affected room. Someone lingering to watch can breathe in acids for no good reason. I’ve worked in labs where one door closed quickly blocked a hallway from hours of residual smell.
A Culture of Readiness Grows Over Time
The most careful teams don’t get that way by accident. They ask for practice drills, share stories, and keep spill kits full and ready to grab. Nobody likes being surprised, and drills mean fewer hands go to Google mid-crisis. Keeping records of past spills and sharing those lessons openly keeps knowledge alive and helps the next person face the problem confidently rather than guessing their way through it.
More Than Just a Chemical: Purity Matters
Para-Toluene Sulfonic Acid, often called p-TSA or PTSA, turns up in labs and factories around the world. While it might not get the headlines, folks in chemical manufacturing, pharmaceuticals, and even some cleaning industries know the stuff well. Its reliability starts at purity. Most of the time, you’ll see PTSA offered at purities close to 98-99%. Plenty of manufacturers trust this range since it keeps reactions predictable and meets high quality standards.
I've handled PTSA in a variety of settings. If you've ever waited on a shipment, you pay attention to details on purity — even a small difference can throw things off. For example, in organic synthesis, impurities cause side reactions, leading to unpredictable results. This usually burns both time and raw materials. Companies set specifications tightly, often pulling from peer-reviewed research and years of practical industry knowledge. This number—typically above 98%—didn’t just come out of thin air.
Why Purity Levels Stick to 98-99%
Let’s talk straight: less-than-optimal purity can break trust and disrupt results. Selling or buying a batch at 95% means you inherit a whole mess of sodium salts, moisture, or byproducts. These leftovers skew test results, damage equipment over time, and risk safety in industrial applications. Even in settings where the margin of error seems generous, I’ve watched whole processes get shut down after a contaminated batch.
Those in procurement track their suppliers based on their ability to hit these benchmarks lot after lot. A spec sheet that says “98%, minimum” signals the manufacturer stands behind the process and monitors every step—from starting materials to final drying. A poor-quality additive creates headaches for every downstream user, not just chemists but managers and technicians, too. Consistency in purity secures repeat customers, not just legal or regulatory compliance.
What Drives Purity? Real-World Practice
In the real world, the cost of producing higher-purity PTSA comes with returns that go beyond paperwork. Clean product means clean reactions, better yields, and safety on the production floor. From my experience, a pharmaceutical company will walk away from a supplier who doesn’t control purity as a rule, not as a suggestion. Their production lines won’t tolerate uncertainty.
Facts bear this out: a 2022 report by a major chemical market research firm shows nearly every large supplier in Asia, Europe, and North America offers PTSA in the 98-99% range. They label the rest as technical grade, which gets steered to industries with less demanding needs. Lower purity might find a home in simple applications—maybe in cleaning—but even there, too many impurities risk corrosion and tool damage. Higher-grade material, on the other hand, finds its way into medicines and electronics.
Better Purity, Better Industry Outcomes
Sourcing PTSA at the right purity isn’t about chasing numbers for the sake of it. The cost of cutting corners turns up quickly, whether in laboratory failures or lost business. The most reliable suppliers don’t settle for “close enough”—they strive for that tight 98-99% mark for a reason. And folks buying know to ask for certificates of analysis and batch records. I’ve seen a supplier’s reputation grow or shrink on that alone.
Looking ahead, more industries want traceability in supply chains. Companies who invest in consistency—by improving their purification processes, by validating their batch testing—stand to win out. Those who gamble with lower grades may sell once, but they rarely turn one-off sales into long-term relationships. For PTSA, and for the people who depend on it, purity isn’t just a technical matter; it’s the foundation of trust and performance.