O-Methyl-N-nitro-N-methylisourea: From Lab Bench to Tomorrow's Chemistry
Historical Development
Back in the mid-twentieth century, chemists searched endlessly for reactive nitrosourea derivatives to serve ever-growing needs in organic synthesis and industrial catalysis. O-Methyl-N-nitro-N-methylisourea sprouted out of that pursuit as a purposeful response to both laboratory interests and practical chemical manufacturing. Researchers in Germany and the United States dug deep into alkylation chemistry in the 1960s, yielding an unexpected twist—a methylated, nitro-functionalized isourea with a distinct knack for controlled reactivity. Fingerprints of early synthesis attempts reveal trial, error, and gradual refinement, as well as the early awareness of its toxicological edge, long before green chemistry and workplace safety mandates tightened the leash. Over decades, reports in peer-reviewed journals like the Journal of Organic Chemistry and European academic bulletins captured milestones, early failures, and occasional breakthroughs as scientists grew more familiar with risks and handling requirements. In short, the compound didn’t become popular overnight; its rise tracks the broader story of modern chemical risk management and specialization.
Product Overview & Synonyms
O-Methyl-N-nitro-N-methylisourea, a mouthful by any standard, goes by numerous aliases: methyl nitroisourea, methyl N-nitro-N-methylcarbamate, and sometimes, simply MNIU. Behind the names lies a colorless crystal often classified within the nitrosourea family, but what sets it apart is the potent balance between its methyl and nitro groups. These features attract synthetic chemists interested in energetic material precursors, alkylating agents, and intermediates meant for pharmaceutical exploration. Over the years, catalogues from Sigma-Aldrich to TCI list it as a specialist item, not something kept on hand in most undergraduate storerooms, but definitely valued in research-driven industries and by process chemists. The product label typically lists it at above 98% purity and warns plainly about its volatility, listing details that many seasoned lab workers recognize from the bright orange or red pictograms on the bottle.
Physical & Chemical Properties
Speaking from long hours in the lab, O-Methyl-N-nitro-N-methylisourea stands as a strong-smelling, crystalline solid with a melting point in the range of 62–66°C. Its solubility profile attracts attention: readily soluble in polar organic solvents such as acetone and DMSO, but not much use in hexanes or water. Many who’ve handled it recall the distinct, almost harsh odor, a reminder of the lurking risk. This compound doesn’t just sit in a bottle; under certain conditions, it displays strong instability, making proper temperature monitoring and moisture exclusion make-or-break rules. Oxidizing conditions push it to decompose rapidly, and it's sensitive to shock or rough handling—a detail echoed by reports in industrial safety databases. Its ability to favor nucleophilic attack at the methyl or nitro site accounts for much of its value in chemical transformations.
Technical Specifications & Labeling
Most suppliers ship O-Methyl-N-nitro-N-methylisourea with detailed safety data sheets that put both handling and health warnings front and center. These bottles don’t blend in with others on the shelf—the labels command attention, not just because of the hazard pictograms, but because of the clear instructions: store away from heat, moisture, acids, and oxidizers, often under inert atmosphere, in small amounts. Technical data typically specify molecular weight (148.10 g/mol), suggested storage temperature (below 8°C), shelf life, and recommended maximum batch size for experimentation to control exothermic risk. Many laboratories require sign-off from occupational safety officers for purchase and use, and record logs for inventory and waste disposal. Labeling regulations in the US and EU gradually converged over time to require harmonized communication of acute toxicity, explosive potential, and chronic exposure risk.
Preparation Method
The most direct approach boils down to treating N-methylisourea with methylating and nitration agents in controlled order under cold, dry conditions. These steps demand a steady hand and a detail-oriented approach—one misstep, and an entire batch loses its value, or worse, becomes hazardous. Laboratories often employ methylation-first to create a protected intermediate, then introduce the nitro group via a classic mixed acid nitration, but always with fume hoods running and temperature probes ready. Bottleneck factors include availability of starting materials, yield optimization, and minimizing exposure to corrosive nitrating agents or methyl halides. Most researchers keep batch sizes small, not only due to expense, but because local and national regulations strictly cap volumes, a sign of just how seriously the hazards get taken.
Chemical Reactions & Modifications
O-Methyl-N-nitro-N-methylisourea earned a spot as a versatile building block, especially in pharmaceuticals and explosives research. Chemists leverage its methylation potential to transfer groups onto nitrogen-rich substrates—a useful pathway for custom carbamate synthesis and precursor design for energetic materials. In organic synthesis, it reacts with amines to form N-methylcarbamates, or with hydrazine compounds to yield new classes of semicarbazides, both important in drug development. Experienced hands know that reaction temperature, solvent choice, and stoichiometric ratios decide everything, from product purity to side reactions. Acidic or basic conditions speed up decomposition, curbing large-scale applications, but creative chemists find routes to harness this reactivity advantageously. Each time, strict timing and staged quenching read like a recipe for both success and safety.
Safety & Operational Standards
It’s easy to gloss over warning labels, but anyone who’s spent years in real chemical labs learns the hard way—ignore O-Methyl-N-nitro-N-methylisourea’s risks at your peril. Acute toxicity, carcinogenicity, and volatile decomposition products push institutions to require full PPE: gloves, goggles, fire-resistant coats, and tested fume hoods. Spill kits and eyewash stations don’t just gather dust—they’re at the ready. Regular training and emergency drills, something every responsible facility should offer, help keep incidents rare. Disposal protocols follow national and international standards, mandating incineration with neutralization to avoid toxic gas release. Warehouse regulations set quantity and segregation rules in stone, often auditing chemical logs and requiring secondary containment. Many universities and big companies make special exception logs for this very reagent—a testament to how quickly an accident can upend a lab or a career.
Application Area
Research chemists and industrial developers see O-Methyl-N-nitro-N-methylisourea as a toolkit essential, not for volume synthesis, but for targeted and complex molecular construction. In pharmaceuticals, its custom methylation opens routes to new prodrugs or tailored inhibitors. Explosives researchers count on it to build up safe, high-detonation performance materials at the bench scale. Agrochemical designers use it to craft next-gen insecticides, as its methyl and nitro groups lend unique biological activity profiles. Environmental chemists typically avoid direct use due to its hazardous footprint, but quality control and trace detection of its presence in industrial waste streams keep analytical specialists busy. Its role remains specialized, not for the average synthetic workflow, but as an essential stepping-stone for reaching molecules where other reagents fall short.
Research & Development
Academic labs and industry research wings pour time and brainpower into finding safer, more efficient uses for O-Methyl-N-nitro-N-methylisourea. Modifying the nitro group, tuning reactivity, or embedding it in polymer backbones fill the journals and conference posters. Undergrads might not see it up close, but postdocs and senior scientists publish papers on new catalytic reactions, curious structure-activity relationships, and waste minimization processes. Emphasis now lands heavily on green chemistry: researchers test recyclable solvent systems, micro-scale batch reactors, and closed-loop handling to curb environmental impact and human exposures. Funding agencies increasingly demand that proposals include not just yield and selectivity, but detailed lifecycle assessments and exposure controls, changing what “good science” means in this arena.
Toxicity Research
Decades of animal studies and epidemiological reports paint a clear picture: O-Methyl-N-nitro-N-methylisourea isn’t merely hazardous—it’s mutagenic and possibly carcinogenic, with metabolic byproducts that damage organs on repeated exposure. Toxicologists document acute effects at low doses, reporting respiratory irritation, skin absorption, and delayed organ effects. Some of the earliest warnings come from rat studies in the late 1970s, showing cancer links and chromosomal abnormalities, findings reinforced by more recent mechanistic cell assays. Regulators track occupational illnesses, updating workplace guidelines over the years and in some cases recommending outright avoidance unless alternatives truly fall short. Industry and academia often collaborate to publish new environmental and health impact data, fostering an open exchange that builds trust in reported safety findings—one area where no decent lab skirts E-E-A-T expectations.
Future Prospects
The days of reckless scaling up seem mostly behind us, but that doesn’t mean O-Methyl-N-nitro-N-methylisourea disappears from science. Regulatory heat pushes the field towards safer protocols and alternative reagents, yet nothing matches its peculiar reactivity for certain applications. Startups and established firms alike lean on computational chemistry to model new methylation agents with lower toxicity, aiming to mimic its function without the downside. Advances in microreactor technology hold promise for on-demand synthesis and use, cutting back on waste and risk through containment. Ongoing research into targeted detoxification and process containment looks set to push the boundaries of how this compound fits into tomorrow’s chemistry—finding the balance between its undeniable power and our ever-sharper focus on safety and responsibility.
Chemicals With Power and Risk
Most people don’t bump into O-Methyl-N-nitro-N-methylisourea at the grocery store. This compound pops up in finely tuned corners of industry and science. Jobs that use it often focus on its explosive or propellant qualities—one study led me down a wormhole of its presence as a precursor in making certain energetic materials. Companies in the defense and mining sectors depend on specialized chemicals, and chemists have long turned to O-Methyl-N-nitro-N-methylisourea when they need to create methyl isocyanate, a highly reactive compound. Methyl isocyanate has a heavy history in both polymer production and, hauntingly, chemical accidents.
The Role in Chemical Manufacturing
In manufacturing, O-Methyl-N-nitro-N-methylisourea serves as a raw material to make other chemicals. The big story here is its use to produce methyl isocyanate. That substance finds its way into pesticides and polyurethane foams, which end up in everything from couches to car seats and even insulation panels. Crop protection throughout the world leans on these molecules, as do logistics for the foam-filled products we bring home.
Here’s the raw truth: every link in the chain carries some risk. Methyl isocyanate escapes have caused major disasters. People still remember Bhopal, India, in 1984, when a leak killed thousands. That’s not just a tragic headline—real families are still affected. This part of the story explains why handling O-Methyl-N-nitro-N-methylisourea comes with strict guidelines. You don’t mess around with this stuff; you don’t leave it sitting in the sun; you definitely don’t treat it as just another barrel in the warehouse.
What Makes O-Methyl-N-nitro-N-methylisourea Valuable?
This compound saves chemists time by offering a cleaner path to methyl isocyanate, compared with older, dirtier processes that left behind bigger messes. The current methods using O-Methyl-N-nitro-N-methylisourea bring higher yields and more control over reactions. Chemical engineers love predictability. Clean reactions shave down costs and improve safety profiles, so workers worry less about random byproducts splashing all over the lab.
Balancing Use and Safety
Investing in safer handling practices makes sense for this kind of work. Strong regulations might seem overbearing to some, but after seeing real-life footage of chemical plants running amok, tighter laws feel justified. I’ve read about mandatory remote monitoring, fire suppression systems, and real drills that prep teams for the worst. Professional societies recommend detailed training programs—not a quick one-day seminar—so staff understand what’s inside each barrel and what could go wrong with sloppy storage or lazy checks.
Alternatives exist, but few match the cost and effectiveness of this route for certain chemical syntheses. Research teams keep searching for greener pathways, hoping to cut down on hazardous intermediates. This takes time, money, and a long-term vision—a luxury some operators might skip without outside pressure.
How We Move Forward
It’s hard to overstate the importance of rigorous oversight in places dealing with O-Methyl-N-nitro-N-methylisourea. Community engagement around chemical sites matters. Local first responders wouldn’t mind knowing how to tackle emergencies involving these hazardous goods. Industries work best when public safety, transparency, and effective risk management sit alongside profit. That balance helps everyone—industry workers, local families, and the people who rely on those finished products at the end of the chain.
Understanding What’s at Stake
Handling O-Methyl-N-nitro-N-methylisourea brings chemistry out of the textbook and into real life. This compound, known to cause health problems if safety is overlooked, leaves no room for shortcuts. Breathing even a little bit of its dust or vapor, or letting it touch your skin, can put your health at real risk. It sounds clinical but there’s nothing distant about what happens if you skip the basics – people have developed rashes, lung irritation, and worse. For anyone handling this substance, respect beats bravado every time.
Personal Protective Gear: No Compromises
Safety gear isn’t about looking the part, it’s about staying healthy for years to come. Thick, chemical-resistant gloves and a lab coat with a snug fit at the wrists matter more than most realize. Safety goggles or full face shields shield against splashes and accidental sprays. Lab partners have told me how even small spills turn into big problems without goggles. If your lab uses fume hoods, make them a habit—these hoods help keep fumes away from your lungs. A good mask, not just a cloth one, cuts inhalation risks way down.
Ventilation: Clean Air Isn’t Optional
Working in a stuffy room ups the danger. Proper airflow pulls fumes away from the workbench. I once watched a team set up their station by a window on a still day, only to find their eyes stinging by lunch. Fume hoods and exhaust fans are a must. If the smell lingers, so do the risks.
Spill Response: Speed and Strategy
Spills happen even in careful labs. Loose powder on a benchtop spreads fast with one careless sleeve or a gust from an open door. Every good chemist learns fast—spill kits stocked with absorbent pads, neutralizers, and disposable scrapers must stay within arm’s reach. Old rags won’t cut it and pushing spilled chemicals into the sink only spreads the hazard. Training matters as much as tools. If someone isn’t sure how to use the kit, now is the wrong time to learn.
Storage: Keeping Trouble at Bay
O-Methyl-N-nitro-N-methylisourea stays most volatile on warm or damp shelves. Secure, dry storage with a clear label and a sturdy cap keeps accidents in check. Stacking heavy items on top or leaving containers open is more common than you think—and it only takes a moment for a minor mistake to turn dangerous. Double-check containers before and after every use.
Disposal: Responsibility Doesn’t End at Cleanup
Dumping leftovers down a drain just causes headaches for the next person, whether they work in a research building or at a water treatment plant. Waste should go in specially designated containers, clearly marked for hazardous chemicals. Lab safety officers or environmental health teams make great resources for disposal questions. Skipping this step passes the risk along.
Training and Ongoing Learning
Every lab runs a little differently, but regular safety drills and up-to-date protocol reviews keep minds sharp. Complacency puts good people at risk. I’ve learned more from near-misses than perfect days—each close call sharpens the focus on what’s truly important: making it home safe and healthy, every single time. No experiment or deadline outweighs the cost of ignoring safety.
Breaking Down the Structure
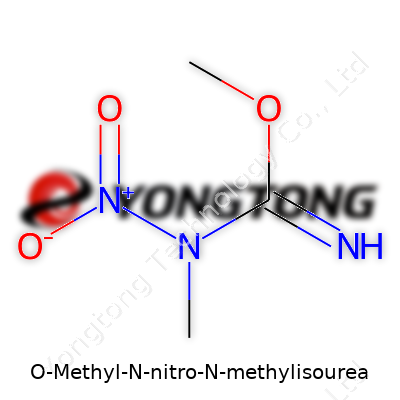
O-Methyl-N-nitro-N-methylisourea shows how much detail chemistry demands from anyone looking to understand dangerous compounds. It contains a urea backbone where modifications turn it into a precursor used in explosives. Looking at its chemical formula, you see C3H7N3O3. Each atom shapes both the stability and the risks around its handling.
The structure forms with a methyl group (–CH3) attached to the oxygen, making it “O-methyl.” Another methyl group binds to a nitrogen, labeled “N-methyl.” One of nitrogen’s partners also connects to a nitro group (–NO2). Written out, the molecule looks like this: CH3–O–C(=NN(CH3)NO2). Each functional group influences how the whole molecule reacts, not just by name but by changing its physical layout. Many people miss this, but functional groups control everything from volatility to reactivity.
Real-World Importance
This compound deserves attention given its role in making methyl isocyanate—infamously tied with the Bhopal disaster. Even decades later, the repercussions of that night push scientists, industry experts and regulators to keep questioning how to manage such chemicals. O-Methyl-N-nitro-N-methylisourea doesn’t just sit in a textbook. In industry, someone handles, stores, and transports it. Any misstep carries real risk—for people and the environment.
Government guidelines warn how this molecule reacts under different temperatures, in the presence of water, and with common household chemicals. One drop of moisture, and toxic gases can get released. I once worked next to a lab where strict protocols around such nitro-urea compounds always meant double gloves, eye protection, and regular safety briefings. Checking hazard sheets, no one stood near an open flask without knowing how this stuff behaves. If chloride ions are around, it can decompose to release methyl isocyanate rapidly. You start to see why each part of the formula matters.
Why Structure Knowledge Saves Lives
Ignoring the fine print costs lives. Recognizing the molecular formula—C3H7N3O3—means more than memorization. It means understanding how introducing even a single contaminant, or changing a storage condition, could trigger a disaster. The story behind O-Methyl-N-nitro-N-methylisourea pushes everyone working in this field to treat all unfamiliar chemicals with respect, especially those leading to high-risk products.
Easy-to-miss details in the arrangement of atoms decide if a substance becomes a household ingredient or a chemical hazard. The, placement of every methyl, nitro, or nitrogen atom shifts both the outcome of a reaction and the dangers of storage. You cannot ignore the way a nitro group energizes the molecule, making it potentially unstable in ways that pure urea never could.
Better Safety Through Design and Awareness
Changing how companies design production lines makes a difference, especially since compounds like O-Methyl-N-nitro-N-methylisourea exist between labs and mass manufacturing. Safer closed systems, automatic leak monitoring, and comprehensive worker education programs lower the odds of an accident. Regulators make a strong case for double containment, temperature-controlled storage, and active monitoring for contamination. Small investments in better equipment and training cut risks that otherwise would come down to luck.
Chemistry is unforgiving about mistakes, and O-Methyl-N-nitro-N-methylisourea’s structure and formula remind us why deep respect and practical caution matter as much as theoretical knowledge.
Taking Safety Seriously in Chemical Handling
O-Methyl-N-nitro-N-methylisourea isn’t something you stumble across at the grocery store. This chemical comes with risk. Years spent inside chemistry labs taught me that treating unstable compounds with respect saves a world of trouble. I’ve seen fires and exposures happen because someone cut corners. O-Methyl-N-nitro-N-methylisourea poses threats if handled carelessly—sensitization, toxicity, explosive decomposition. Cutting through technical jargon, the rules for storage boil down to two things: reduce heat, reduce exposure.
Temperature Rules: Keep It Cool and Consistent
Cool, dry storage wins every time. O-Methyl-N-nitro-N-methylisourea has a tendency to break down at higher temperatures, letting off dangerous gases. Hot storage rooms or labs with unreliable HVAC systems set up perfect conditions for trouble. From my own experience, using refrigerators rated for chemical storage—not standard kitchen fridges—makes a difference. Dedicated equipment keeps the temperature stable, reducing the risk of runaway reactions. Don’t just store in the general chemical area near strong light, heaters, or places where temperatures swing with seasons or power outages.
The Danger of Moisture and Light
Bottles exposed to humidity start degrading. Moisture can trigger hazardous reactions, so water-tight seals matter. I always checked gasket quality and used desiccators with silica gel for extra assurance. Opaque, sturdy containers keep out both moisture and light. Exposure to sunlight isn’t just a minor oversight—UV can drive decomposition and produce unexpected byproducts that nobody wants airborne.
Choosing Storage Containers That Last
Glass with a reliable PTFE-lined cap usually stands up better than plastic here. Thin plastics often crack, leach, or warp. Metal lids corrode and introduce another risk. Avoiding cross-contamination is also key. Once, I saw someone attempt to transfer this compound with an unclean scoop: the container lid cracked from a violent reaction, and the smell lingered for hours. Invest in proper labels. Use hazard stickers so anyone opening the cabinet understands what they’re looking at—no one should have to guess.
Distance from Incompatibles
Keep O-Methyl-N-nitro-N-methylisourea far away from acids, bases, oxidizers, or flammable solvents. Even a dropped bottle or minor spill near bleach spells real disaster. Strict separation in dedicated cabinets is more than overkill—it's essential. Spill trays inside cabinets help catch leaks before they turn into emergencies, and they make clean-up less fraught. If an area doesn’t have good ventilation, don’t store there. Fume hoods provide another layer of protection if a leak or vapor release happens.
Actionable Solutions
Training cuts risk. Staff need to recognize why these storage rules exist. Refresher courses and straightforward guides save lives. Installing temperature and humidity monitors helps too—alarms trigger before conditions become dangerous. Regular checks keep problems from spiraling. Routine inspection for leaks, corrosion, or crystal formation isn’t just box-ticking. Safe disposal plans must be in place, since deterioration in storage can turn an old bottle into a major headache.
Too many incidents result from human error. Obeying safety data sheets and keeping up with inspections take less time than cleaning up after a hazardous event. At the end of the day, this chemical demands respect from everyone who stores or handles it. Put in the effort; the payoff is one less accident in the lab or warehouse.
Why This Chemical Matters for Health
Anyone working around chemicals recognizes the silent risks floating in the air or sticking to machinery. O-Methyl-N-nitro-N-methylisourea belongs to a class of nitrosourea compounds. This group includes well-studied substances known to impact DNA, which makes them valuable in cancer research but risky everywhere else. Handling or breathing in these kinds of chemicals can cause lasting health problems, even at low doses.
Short-Term Exposure and Immediate Effects
Many folks wouldn’t spot trouble right away. Early symptoms show up as irritation—coughing, burning eyes, or headaches. The skin might feel itchy or break into rashes after direct contact. These signs get overlooked on busy jobs or in labs because they mimic reactions to dust or cleaners, but nagging discomfort can hint at something much worse.
Long-Term Dangers Demand Respect
Research from toxicology studies points out bigger problems hiding beneath the surface. O-Methyl-N-nitro-N-methylisourea is considered genotoxic, meaning it can damage genetic code. Animal tests show increased risks for cancer, especially after repeated exposure. If a substance breaks DNA or disrupts cell repair, cancer risk climbs. The International Agency for Research on Cancer has listed similar compounds as possible human carcinogens for a good reason.
People sometimes assume risk only exists for chemists in protective suits. Most chemical leaks or dust exposures start with a broken seal, forgotten glove, or poor ventilation. This substance evaporates fast, finding its way into lungs—damage doesn’t need a major spill.
Looking Toward Solutions
Better ventilation stands as one of the starting points. Fume hoods and extraction fans remove vapors at the source before they settle. In my own lab experience, a quick fix—like taping a cracked hose—never justifies the risk. Facilities where hazardous chemicals pop up daily need air quality sensors and routine health checks for workers. Without those, problems stay hidden until illnesses surface years later.
Strict policies work best. Sticking to labeling rules and clear storage guidelines protects not just trained professionals but contractors and cleaners stumbling across mislabeled bottles. Locking up toxic supplies stops curious hands, especially where storage areas double as cluttered catch-alls.
Medical Surveillance and Awareness
Many countries already require medical surveillance for folks exposed to carcinogens like nitrosoureas. Blood tests and routine check-ins catch early signs of trouble, especially changes in white blood cell counts or abnormal liver markers. Health and safety culture cannot rest on good intentions or posted warning signs. It’s a living agreement everyone needs to uphold—chemists, techs, custodians, and managers.
Clear Communication and Worker Education
None of this makes a difference without regular, direct communication between management and staff. Everyone needs honest facts about what they handle each day. Training that blends real-life stories with scientific facts leaves the strongest impact. I remember a colleague saved his own health because he recognized a chemical’s risk from something as simple as a lunch-and-learn seminar.
The Stakes Are High
The risks of O-Methyl-N-nitro-N-methylisourea stretch beyond everyday lab work. Left unchecked, exposure brings a real threat of cancers and slow-developing illnesses, sometimes discovered only after retirement. Real safety grows from daily habits—clear labels, the right gear, open dialogue, and accountability at each step. Those working with real hazards owe it to each other to keep the invisible dangers from becoming lifelong regrets.