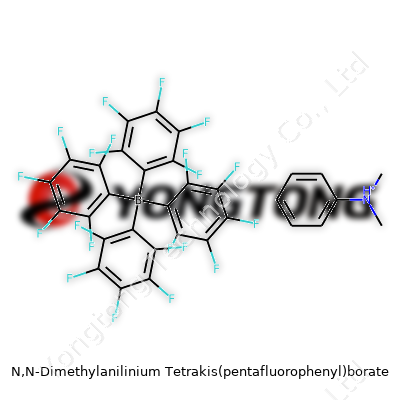
N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate: Deep Dive Commentary
Historical Development
N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate didn’t appear by accident. Chemical innovation often comes from pushing boundaries with old ideas. Developers wanted a compound that could step up as a non-coordinating counterion, so researchers in the late 1990s and early 2000s, searching for better catalysts, started exploring the power of highly fluorinated borates. Back then, standard borates didn’t provide the kind of stability or ionic dissociation that more demanding syntheses asked for. At several conferences, chemists presented how stability and low nucleophilicity in anion chemistry could expand the reach of organometallic catalysis. When scientists combined N,N-dimethylanilinium with tetrakis(pentafluorophenyl)borate, the resulting salt opened doors in a range of catalytic processes, particularly for making polymers and organic electronics. Synthetic chemists learned quickly that this compound doesn’t just fill a niche—it sets benchmarks.
Product Overview
N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate arrives as a pale powder, known for its high purity and utility as a stable, non-coordinating ion pair. Distributed by specialty chemical suppliers in tightly sealed containers, its use sees action in research labs and industrial setups exploring catalytic mechanisms. The salt’s distinctiveness comes from its large, fluorinated borate anion paired with the relatively simple dimethylanilinium cation, which together resist aggregation and unwanted side reactions. This product presents consistency and performance in environments that punish most salts. Researchers value it for its way of supporting reactive intermediates, giving them room to show their true color in solution without interference from counterions.
Physical & Chemical Properties
The physical story for N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate starts with a low melting point (usually between 180°C and 200°C), and it doesn’t like water. Moisture introduces hydrolysis, so you only see it handled with dry glassware. Its large, complex borate side harbors four pentafluorophenyl groups, giving it impressive spatial bulk and fluorine-driven electron withdrawing character. Solubility in organic solvents such as dichloromethane, toluene, or benzene is moderate – you don’t want to force this salt into something polar, as stability unravels. The molecular weight climbs well into the six hundreds (over 736 g/mol for the salt), so lab techs always factor that in for weighing and formulating. As for UV-Vis or NMR characterization, its signals have been used as textbook examples of weakly coordinating anion behavior. Real-life chemical work doesn’t reward guesswork, so having concrete, predictable physical and chemical benchmarks means much fewer experimental surprises.
Technical Specifications & Labeling
Any bottle shipped from a reputable supplier will come with a purity rating, often above 98 percent, batch number traceability, and a thorough labeling that covers not just the chemical identity but detailed handling instructions. Those labels seem tedious but matter once opened on the benchtop. Regular users keep it double-sealed, labelled for anhydrous inert-atmosphere storage. SDS sheets lay out the ground rules: avoid inhalation and contact, don’t let the material contact moisture, and wear standard PPE, including gloves and goggles. Each delivery includes a certificate of analysis, tracking spectral data from proton and carbon NMR, ensuring researchers receive exactly what their protocol asks for.
Preparation Method
Manufacturing N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate involves careful craftsmanship. Researchers start by reacting N,N-dimethylaniline hydrochloride or the free amine with sodium tetrakis(pentafluorophenyl)borate in a dry organic solvent. Filtration and concentration steps follow, with crystalline material isolated under inert conditions to prevent degradation. Each run’s success rides on excluding moisture every step of the way, since the anion’s pentafluorophenyl arms don’t tolerate water. Laboratory synthesis scales easily but never quietly—every glassware glovebox set-up tells a story of the lengths chemists go to protect their product from ambient air and humidity. Robustness comes down to years of practical know-how passed down through research groups.
Chemical Reactions & Modifications
Chemically, N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate acts more as a team player than a soloist. Its primary reputation comes from being a stable supporting ion that doesn’t interfere with cationic intermediates in catalysis. Deploying it in olefin polymerization or hydride abstraction, chemists put its non-coordinating credentials to the test. Analogs have been explored, such as swapping the dimethylanilinium cation for trityl or other onium candidates, but the borate backbone remains central to function. Modifications focus on the substituents of the borate but rarely deliver the same blend of solubility, stability, and inertness. In many catalytic cycles, it hands over the cationic fragment, fading into the background, letting transition metal complexes shine without competition from nucleophilic anions.
Synonyms & Product Names
On shelves and in research papers, N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate can be seen under several guises, such as N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate, DMA-BArF, or its extended IUPAC name, with CAS number 147262-43-3. Suppliers label it either as DMA-BArF or the full chemical name, making catalogue browsing a little less confusing for seasoned chemists who have to hunt down rare specialty chemicals regularly. Familiar acronyms and shorthand grow out of years of use in grant applications and lab notes.
Safety & Operational Standards
Anyone handling this salt respects its need for dry conditions and specialized equipment. Standard laboratory PPE applies: nitrile gloves, safety glasses, and careful planning. Spill response doesn’t ask for anything exotic, but quick clean-up and proper waste disposal protect both the chemist and the environment. The pentafluorophenyl groups impart stability, yet no one ignores the risk posed by borate salts if mishandled. Many research labs enforce glovebox or Schlenk line work as a standard. The SDS details spill procedures, storage advice, and first aid, so no researcher can claim ignorance after an accident. Responsible chemists prioritize training and reminders because one careless moment can destroy a batch and add unwanted danger to a day’s work.
Application Area
Spectacular advances in organometallic chemistry and polymer science owe plenty to non-coordinating anions like this. In metallocene and post-metallocene olefin polymerizations, N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate acts as more than a spectator. It activates catalyst systems, enabling creative control over polymer architecture no one dreamed of in early Ziegler-Natta chemistry. Development of conductive polymers, OLEDs, and fine chemicals has seen this salt become a central figure in generating highly reactive carbocations, silylium ions, and other fleeting intermediates. Without its stabilizing touch, many advanced materials projects would grind to a halt. Medicinal chemists and materials scientists take advantage of its clean background in sensitive transformations, finding value where conventional anions fail.
Research & Development
Current R&D projects feature this salt in screening new homogeneous catalysts and for the generation of superacid-like environments. Electrochemical studies and photoinitiator development tap into its abilities to generate persistent cationic species. Chemistry conferences showcase results where switching from conventional borates to the tetrakis(pentafluorophenyl)borate system turns previously mediocre yields into best-in-class results. At the academic edge, researchers test how modifications to either cation or anion affect not just reactivity, but selectivity and process scalability. Even undergraduate chemistry projects see the salt make an appearance in organometallic demonstration experiments because it behaves predictably, allowing students to focus on learning principles rather than troubleshooting wayward side reactions.
Toxicity Research
Toxicological profiles for N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate continue to grow. Data so far suggest that, though the salt’s components are not acutely toxic in tiny research quantities, long-term exposure risks or breakdown products have yet to be fully understood. The cation comes from a parent amine known to cause irritation, but its borate partner’s wider biological impact—especially the perfluorinated phenyl groups—draws strong environmental concern. Regulators and safety officers want more studies looking into persistence and bioaccumulation, as perfluorinated compounds often resist degradation and can persist in groundwater. Labs minimize emissions, capture residues, and push for greener synthesizing protocols wherever possible. Until broader studies close the knowledge gap, prudent researchers reduce exposure and treat every protocol with environmental care.
Future Prospects
There’s little doubt about the long-term impact of N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate in advanced synthetic chemistry. As more researchers aim for higher efficiency and selectivity, non-coordinating borates take a front seat in developing next-generation materials and pharmaceuticals. With regulatory scrutiny sharpening around PFASs and related compounds, the chemical industry faces calls to design more biodegradable or less persistent variants while keeping core performance. Expect new protocols that replace legacy solvents with green alternatives, or new anion frameworks that keep stability and low reactivity without relying as heavily on fluorinated aromatics. For early-career chemists and industry veterans alike, keeping up with safety training, green chemistry initiatives, and regulatory shifts will always matter just as much as mastering the next catalytic breakthrough.
The Compound that Gets Chemistry Moving
Chemists don’t usually get too excited about long names, but N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate—or DMAPF5B—has carved out a place on the lab bench. The primary draw sits in its role as a transfer agent for borate anions, but the real story comes from what that means. Scientists use this compound to generate extremely reactive cations—especially carbocations and metal complexes. These cations play a big part in complex organic transformations and catalysis that typical salts can’t handle. So, DMAPF5B acts like a backstage kit for those bold chemical reactions people depend on in synthesis and advanced material science.
No Ordinary Salt: Non-coordinating Support
I remember handling ordinary salts early in my chemistry training, always limited by anions that stuck around and messed with the star players—reactive cations. DMAPF5B offers a way past that. Its huge, flexible borate anion doesn’t play favorites with the metal or organic cations being studied. Instead of sticking, it gives those cations the freedom needed to show their true properties and reactivities. That’s vital for exploring how certain metals activate small molecules or for making highly targeted pharmaceuticals.
Tuning Catalysts for Breakthroughs
Researchers in industry and universities turn to DMAPF5B in pursuit of better catalysts. Manufacturers, for example, use it in olefin polymerization reactions. By generating “naked” metal cations with this borate salt, scientists create highly active catalysts. These are the engines behind important materials: special plastics, high-performance rubbers, and certain fluorinated polymers. Academic chemists, too, uncover new ways to manipulate carbon-hydrogen bonds or assemble rings and chains—all because the anion simply doesn’t interfere. That translates to higher yields, quicker reactions, and less waste.
Challenges and Clean Hands
Every time I have seen DMAPF5B used in a lab, people treat it with respect. Pentafluorophenylborates, though valuable, do not come cheap. Synthesis and handling pose hurdles since borate salts sometimes react with moisture in the air. Proper storage and dry techniques are standard. There’s a learning curve for newcomers, but the rewards often outweigh the cost and effort. Using cleaner chemistry leads to less complicated product mixtures, which reduces headaches in purification and safety testing. Laboratories seeking speed and purity know the value of this specific salt.
Pushing Green Chemistry Forward
Green chemistry matters more every year—both inside the lab and on the production floor. DMAPF5B makes a difference by supporting reactions that avoid more hazardous byproducts. When classic anions gum up the works, researchers end up with more side reactions and tougher clean-up. With tetrakis(pentafluorophenyl)borate on hand, processes get simpler, which cuts down on solvent use and waste streams. That cleaner chemistry fits with stricter regulations and social pressure for responsible science. In my view, every step in that direction counts, whether you’re discovering medicines or scaling up new battery materials.
Science Finds Power in the Right Tools
I’ve learned over the years that small changes in the chemistry toolkit bring outsized impact. N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate lets chemists see and use reactivity that would otherwise get buried or blocked. It’s not household chemistry, but for anyone solving problems in modern synthesis or catalysis, this salt turns tough experiments into real progress. The future of cleaner, sharper, and more efficient chemistry owes a lot to tools like DMAPF5B.
Why Proper Storage Is Critical
Handling chemicals like N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate never feels routine. It’s not simply another lab supply sitting on a shelf. This compound, used in catalyst preparation and as a non-coordinating anion, brings both benefits and hazards. University years showed me how even careful students can overlook bottle labels or underestimate reactivity, which leads to headaches later. That slipshod attitude risks losing product, damaging equipment, or even causing injuries.
Understanding What You’re Working With
The long name alone signals complexity. Here, you’re dealing with a salt often sold as a fragile powder, moisture-sensitive, yet necessary for work on organometallic chemistry or advanced synthesis. This compound reacts easily with water, breaking down and ruining the yield. A friend of mine once watched pricey borate salt turn useless from a simple cap left loose overnight. That single mistake cost more than just money; it meant starting a project from scratch.
Best Storage Habits: What Actually Works
No one gains from fancy talk about “air-tight environments” unless the advice is practical. Glass containers with tightly fitting, chemically resistant caps do the job. I keep desiccators handy. A desiccator filled with fresh desiccant, often silica gel or anhydrous calcium chloride, adds a layer of protection. Hydroscopic compounds like this borate salt stay stable only if moisture remains below a fraction of a percent. One slip lets enough humidity in to spark breakdown.
Light and temperature throw in their share of risks. Direct sunlight can trigger changes in sensitive organic salts. Ordinary lab shelves quickly turn too warm, especially near windows or radiators. The best solution I’ve found involves a cool, dry storage cabinet, tucked away from sunlight, combined with clear labeling—date received, date opened, and strict expiration checks. Leaving out these simple steps often ends badly.
Lab Reality: Mistakes and Smart Solutions
Skipping basics tempts disaster. Keeping bottles loosely capped, or allowing shared labs to become clutter magnets, opens the door for cross contamination or even toxic accidents. I’ve learned to double-bag containers in zip-top plastic, sealing out stray air during daily lab routines. Nitrogen glove boxes or dry boxes, if the budget allows, provide extra layers of security. For researchers without these tools, investing in smaller aliquots and using only what’s needed cuts down on loss from environmental exposure.
Chemicals with “tetrakis(pentafluorophenyl)borate” in the name rarely belong in household or DIY spaces. Institutional safety guidelines aren’t just paperwork; they follow hard lessons. Eye protection, gloves, and ventilation draw a line between a successful day and a near-miss.
Looking Ahead: Safer Practices and Better Results
The simplest practices—tight seals, cool and dry storage, clear labels—aren’t optional steps. They stop waste, prevent dangerous breakdowns, and protect research dollars. Sharing lessons learned, inside a team or online, builds a better safety culture. Colleagues catching early warning signs, like clumpy powder or bottles sweating inside, protect the entire lab community.
In the end, storing N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate with care doesn’t just protect a chemical. It supports smarter research, respects the challenges of handling sensitive substances, and keeps accidents out of the headlines.
Understanding Why We Respect the Rules
Safety in a lab or on an industrial site isn’t just about following protocol because that’s what bosses or textbooks say. I've learned through direct work with chemicals—one incident involving an improperly stored bottle of concentrated acid and a friend’s ruined jeans—that respect for safety rules keeps everyone from making the sort of mistakes that change lives in a split second. Burns, poisonings, fires—these stories show up in newsrooms and emergency rooms all the time, often because somebody decided to skip a step.
The Big Factors: Routes of Exposure and How They Sneak Up on You
Understanding the path a compound takes into the body changes the way safety measures are set up. Inhalation, for example, doesn’t just mean breathing in visible fumes. Years ago, a local plant technician got sick from a colorless vapor floating well below the odor threshold—no warning sign, no time to realize the risk. That’s why relying on your nose doesn’t cut it. Volatile liquids, fine powders, or even splashes can catch you off guard.
Skin exposure causes plenty of problems too. Some corrosives and organic solvents head straight through gloves that look sturdy enough at a glance. Once, while I worked with simple ethanol, a colleague laughed off a splash that soaked through a glove and came away with a red, itchy burn. It's not just about pain; some compounds trigger allergic reactions or linger in the body.
Personal Protective Equipment: Not Optional
Real protection starts with goggles that fit, not just resting on your forehead. Chemical-resistant gloves—chosen for the specific substance—keep hands safe, but they break down faster than you'd expect. I saw nitrile gloves crumble from a concentrated acid, leaving fingers exposed. Lab coats, closed-toe shoes, and sometimes full face shields round out the gear. Gear only helps if it’s worn right, cleaned regularly, and swapped out at signs of damage.
Ventilation: Making Air Count
Good air movement pulls harmful vapors away. Fume hoods do the heavy lifting where I work, but only if the sash stays low and nothing blocks the vents. Portable fans don't do the trick; they just stir things up. Proper ventilation isn’t fancy—it comes down to keeping hazardous air out of your lungs.
Storage: Don’t Mix Trouble
Chemicals stored together can react in nasty ways. A shelf full of acids next to reactive metals sounds like fiction, but I’ve seen it in crowded storerooms. The result: fires, explosions, toxic gas. Labels matter. Double-checking compatibility charts before shelving a new bottle keeps these disasters from sneaking up overnight.
Training and Emergency Plans: Life Savers, Not Paperwork
Regular, real-world training beats long, boring online modules every time. Watching an instructor handle a spill, seeing how to suit up for cleanup, or practicing a quick eyewash rinse sticks with you when panic hits. Written procedures matter, but muscle memory wins under stress.
Eye-wash stations, safety showers, and spill kits have saved colleagues from pain and long-term injury. Knowing where they are and how to use them keeps a minor incident from becoming a headline.
Better Practices, Fewer Accidents
Safety precautions aren’t just rules—they’re part of the culture in any place that handles dangerous compounds. Listening to science, sharing mistakes openly, and choosing the right tools for the job makes everyone safer. The job gets done, folks go home in one piece, and bad stories stay just that—stories we tell to make sure history doesn’t repeat itself.
Looking Beyond the Formula
Nobody walks into a lab and grabs a bottle without thinking of solvents. Most chemists know that N,N-Dimethylanilinium Tetrakis(pentafluorophenyl)borate (DMA-TfPB) carries a long, complicated name for a good reason—its chemistry isn't straightforward. A big challenge is understanding how this salt behaves once taken out of its bottle, especially in everyday solvents.
Lab Experience Matters
My first run-in with DMA-TfPB came with a project focused on non-coordinating anions. You don’t get far without noticing it looks powdery and crusty in its container. Toss it in water, and nothing impressive happens—it clumps and refuses to dissolve. The borate counterion, loaded with fluoro groups, makes it hydrophobic. Even adding heat barely moves the needle on solubility. So anyone thinking of using home-lab basics like water or ethanol gets disappointed fast.
How the Salt Responds in Different Solvents
Traditional water-based chemistry doesn’t do the trick with DMA-TfPB. I tried methanol and ethanol in the hope that their organic side would play nicely, but the solution stayed cloudy. This pattern tells us that the salt's fluorinated anion stacks the deck against polar solvents. Dipping into acetone or acetonitrile gives a bit more promise. These solvents manage to take up some of the salt, but not in amounts that make life easy for reaction screening or scaling up.
Getting better results required looking at less polar, more aromatic solvents. Toluene and dichloromethane step into the spotlight here. They match up with the non-polar, bulky anion, allowing some room for the cation to break free from the lattice. I still saw undissolved material at room temperature, but gentle warming or sonication coaxed more into solution. Chlorinated solvents like chloroform, and especially dichloromethane, turn out to be reliable choices for dissolving moderate quantities of DMA-TfPB for catalysis, anion exchange, or organometallic work.
Safety and Sustainability Trade-Offs
Switching to halogenated solvents brings new issues. Dichloromethane makes life easier in the flask, but it brings strong health and environmental warnings. Years working in academic and industrial settings drilled into me the importance of reducing exposure where possible. Anyone using DMA-TfPB at scale or frequently across projects dodges unnecessary risk by planning for safer solvent use or engineering controls like fume hoods and double-gloving.
Innovation in the Face of Solubility
A chemist’s toolbox grows by addressing hurdles like these. Recent publications point to attempts using solvent blends, or even designer ionic liquids to pull DMA-TfPB into solution more easily. Some researchers experiment with ligands or co-solvents that encourage better salt breakup. My own trial with a DCM/THF mixture worked surprisingly well, allowing clear, usable solutions for catalysis.
Practical Solutions
Instead of chasing after the impossible and forcing a water-friendly formulation, most routes benefit from thoughtful solvent choice tailored to the problem. Good records and a willingness to experiment make all the difference. Cutting corners on solvent safety to boost solubility just trades one problem for another.
Lab work keeps reminding me that a deep understanding of solvent-salt interactions pays off. DMA-TfPB won’t dissolve in everything, but with some experience, clear guidelines emerge: stick to dichloromethane or toluene for most jobs, explore co-solvent systems if you want to push limits, and always keep an eye on safe practice.
Why Catalysts Matter
Catalysts change the way industries work. In my years touring chemical plants, I’ve watched reactions run hundreds of times faster thanks to a pinch of the right material. In pharmaceuticals, for example, precious metal catalysts like palladium unlock routes to life-saving molecules that would otherwise take days or weeks to build. You see the impact in the speed and the yield—less waste, less energy, better results. The application stretches into almost every process that underpins modern life, from medicine to plastics to fuels.
Applied in Large and Small Scale Synthesis
Take hydrogenation, a step used everywhere from margarine production to petroleum refining. Nickel and platinum serve as workhorses here. Manufacturers rely on these metals to break tough molecular bonds or attach hydrogen in the right spot. Refining crude oil into gasoline involves a precise series of catalytic steps, each with its own specialized material. The result is cleaner fuels and fewer byproducts, which means a smaller impact on the environment.
Organic synthesis classes showed me dozens of ways chemists lean on catalysts. In making flavors or fragrances, acid-based resins steer the formation of esters and ethers without muddying the taste or introducing unwanted side products. On the industrial scale, these choices make all the difference, trimming down reaction times and leaving behind purer batches.
Green Chemistry Steps Forward
Environmental rules put pressure on industries to rethink old habits. The old days of dumping solvents or burning off leftover chemicals are long gone. Catalysis offers real hope here: better selectivity and lower temperatures mean factories slash energy use and vent less pollution. Zeolites, for instance, provide a network of tiny pores that sort molecules by size or shape. Refineries use them to trap only the molecules they want, avoiding costly and dirty side streams.
Enzymes provide another path, especially in food and pharmaceutical production. These biological catalysts give companies a way to work with water instead of solvents, often at room temperature. The result? Cleaner processes and products that earn an easier regulatory pass. Even paper mills have gotten on board, using enzymes to bleach pulp without harsh chemicals.
What’s at Stake
Without reliable catalysis, we pay more for daily products—from soap to vitamins. Factories lose efficiency, and the planet takes the hit. Research from the Royal Society of Chemistry points out that up to 90% of all chemical processes use some form of catalysts. It’s impossible to ignore their reach. The world’s supply of ammonia fertilizer, which feeds billions, relies on the Haber-Bosch process—a reaction made possible only because clever engineers found a way to speed things up with iron-based catalysts.
Improving the Future
Investment in research matters now more than ever. We could use nanotechnology to tune catalysts more finely or recycle metals that are running short. As someone who’s seen the frustration in a lab when a catalyst poisons, gets contaminated, or goes missing, I know innovation in this corner of science saves real money—and sometimes, lives. More collaboration between universities, startups, and big industrial players will make tomorrow’s catalysts cheaper, safer, and less damaging to ecosystems.
Catalysis stays vital in both old processes and new tech. Bringing fresh eyes and steady funding into this arena promises a more efficient, greener, and sustainable future for everyone.