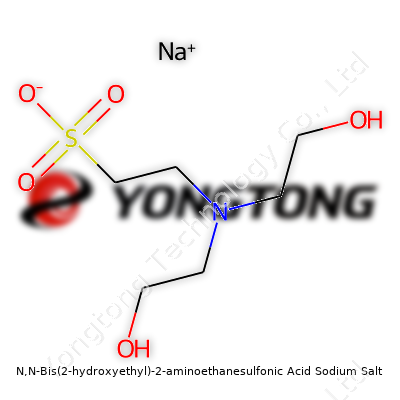
N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic Acid Sodium Salt: A Closer Look at a Quiet Workhorse
Historical Evolution and Breakthroughs
N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid sodium salt did not emerge overnight. Its roots come from the burst of research on biological buffers in the late twentieth century, a time when the realities of enzyme kinetics and protein conformation demanded something better than old-school phosphate and Tris buffers. Pioneering chemists went after molecules that could hold steady against pesky pH shifts and enjoy compatibility with sensitive biomolecules. I remember reading about the early work with sulfonic acid derivatives and realizing how their stability in aqueous solutions answered critical needs in conserving enzymatic activity. Part of the success of this compound comes from its background as a zwitterionic buffer combining biocompatibility with robust chemical resilience.
Product Overview in Real Context
This sodium salt operates mainly in laboratories and industrial settings where precise pH buffering is not just a bonus—it’s a must. From my own time in campus research labs, someone would always have a jar or bottle of this buffer powder. A researcher weighs out a small pile, dissolves it in water, tweaks the pH, and knows their biochemistry experiment won’t get skewed by buffer breakdown. Its sodium salt helps boost solubility, which makes prepping solutions less of a hassle—no endless stirring or mysterious precipitates. In diagnostic assay kits or high-end fermentation gear, this buffer quietly ensures results don’t drift due to environmental changes.
Physical and Chemical Qualities That Matter
Walking into a chemical storeroom, I’ve noticed that this compound’s white, crystalline appearance always inspires trust. It dissolves freely in water, forming a clear solution that won’t foam or clump unexpectedly. The sodium salt’s stability stretches across a broad temperature and pH range, which pays dividends for experiments running overnight or under unpredictable bench conditions. Its low UV absorbance around 260 and 280 nm means minimal interference in protein or nucleic acid quantification. At the same time, its resistance to oxidation and lack of reaction with most biological molecules keeps results reliable.
Technical Specifications & Labeling in Practice
Quality control teams look for high assay values, generally over 99%, with impurity limits spelled out for heavy metals or organic contaminants. Packaging weighs in at everything from tiny glass bottles for research use to multi-kilogram drums for production lines. Labels spell out storage conditions—cool and dry, as usual, and hazard statements highlight minimal but real risks, like eye irritation with accidental contact. Even in a storeroom’s dim light, batch numbers and expiry dates stand out because expiry impacts precision—especially in regulated pharmaceutical work.
Preparation Method and Its Lab Implications
Synthesis often starts with ethylene oxide and aminoethanesulfonic acid units, relying on controlled alkylation and sulfonation reactions. Industrial chemists optimize solvent and catalyst use to raise yield and prevent messy byproducts. In smaller labs without access to commercial suppliers, people sometimes cobble together modest amounts through less sophisticated routes, but that approach often makes purification a headache. Most production avoids excessive byproducts and highlights easily recyclable solvents, reflecting a step toward greener lab routines. Experience has shown that using store-bought, certified product beats battling impurities in home-baked batches, especially with high-stakes or publication-bound research.
Chemical Interactions, Derivatives, and Modifications
Once in solution, this buffer reacts gently with acids and bases during pH adjustments but tends to ignore most biomolecular functional groups. Some specialized protocols add extra modifications—for instance, isotopic labeling for NMR studies or fluorescent tags to follow buffer diffusion in living systems. Researchers have explored esterification, mainly to tweak solubility or generate slow-release forms in industrial fermentation. In my experience, if you keep things clean and stick to water-based systems, you rarely see unexpected side reactions, which means more reproducible data.
Synonyms and Sales Names in the Real World
This compound carries an armful of synonyms, depending on supplier and cultural context. Most labels mention BES-Na or Sodium BES, and chemical texts often marry the long IUPAC name to those simpler tags. Differences mostly reflect regional nomenclature conventions and corporate branding—some Asian manufacturers use transliterations, while Western catalogs lean on acronyms. These naming quirks rarely throw off seasoned lab staff, but students and newcomers can get tripped up if catalog searches turn up synonyms when they expect chemical formulas.
Safety Standards and Everyday Lab Practices
Even widely used biochemicals like this require attention to safety. The dust finds its way to hands or eyes quickly, especially during large-batch prep. Gloves, splash goggles, and smart handling habits keep accidents rare. Spills get scooped up and dissolved in water, then poured down drains only if permitted by lab regulators, who watch for sulfate loads in wastewater. MSDS printouts anchor the safety culture, and regular risk training reminds staff that every chemical, familiar or not, demands respect. From broken bottles to leaky sachets, lab stories show one thing—good safety habits trump any technical guideline.
Key Applications in Science and Industry
The real value of this sodium salt often appears in protein purification, cell culture, and enzyme assays. My own brief stint in a pharmaceutical lab showed how this buffer kept antibody fragments happy and stable during purification columns. In environmental testing, its pH control powers heavy metal analysis. Downstream, breweries and biotech firms use it to safeguard fermentation cultures from pH swings that would ruin batches. Companies developing diagnostic kits turn to this familiar buffer because it integrates easily into automated workflows, giving confidence to users who lack the time or resources to troubleshoot buffer mysteries.
Research & Development Driven by Real Needs
Every so often, research teams look for new ways to use or tweak this buffer salt to expand its toolkit. Scientists studying cryopreservation appreciate its low toxicity and compatibility with living cells. People engineering novel biomaterials dig into its unusual hydrogen bonding potential, hoping to stabilize fragile complexes. With the rise of high-throughput assays and custom analytical techniques, modifying buffer properties for ultra-specific uses now appears on grant applications. Many groups focus on greener synthesis—replacing harsh reagents or finding ways to recover sodium or organic precipitates. Students tasked with finding new buffer systems regularly compare results with this compound, turning up gaps and sparking fresh investigation.
Toxicity Studies and Practical Considerations
Long-term experience shows low acute toxicity when handled with common sense. Animal studies and cellular assays reveal little in the way of mutagenicity or chronic harm, meaning research animals and cell lines survive even extended exposure. Even so, repeated skin contact dries skin, and nobody likes a dry, cracked thumb after hours of buffer prep. Regulatory bodies request clear documentation about disposal and suggest best practices for accidental ingestion or inhalation. These reports help companies assure regulatory bodies and the public that the chemicals supporting breakthrough research don't jeopardize long-term health or the environment.
Paths Forward and Future Directions
Research groups and industrial chemists constantly hunt for improvements. With sustainability pressure rising, greener synthetic pathways top agendas, and many labs look at waste streams and solvent use to trim the carbon footprint. New applications pop up in mRNA vaccine platforms or next-gen diagnostic devices, calling for finer tuning of buffer purity and performance. I see growing collaboration between chemists and environmental scientists to balance performance with lifecycle analysis. Investment in digitized tracking of buffer quality offers the promise of faster troubleshooting if sudden batch variations appear. The next few years should bring not only cleaner manufacturing but also smarter, more responsive use in research, diagnostics, and production lines.
A Buffer with a Critical Role in Labs
Walk into any life sciences lab and you’ll find buffers stacked on the shelves. N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic acid sodium salt, known by the shorthand BES-Na or BES Sodium, holds an important spot in this category. The word "buffer" might sound technical, but its meaning is simple. Buffers keep things steady. They protect the pH level in a solution, keeping it from swinging up or down. If you’ve ever baked bread, you know small changes in ingredients can make or break a loaf. The same goes for chemical reactions — pH matters a lot.
BES-Na isn’t the most famous buffer out there, but it’s picky about what it does best. Labs pick BES-Na for situations where stability across a middle pH range is needed—often between 6.4 and 7.8. I spent years in a university research lab working with mammalian cell cultures. We leaned on BES-Na to prepare our growth media, looking to hold that pH right in the sweet spot needed for the cells to grow and behave like they do in the human body. If the pH slides off target, the results can turn sloppy in a hurry — cells die, proteins change shape, data heads south.
Beyond the Petri Dish
The uses for BES-Na stretch far past cell culturing. Many diagnostic tests, such as blood analysis equipment and biochemical assays, need pH levels to stay put during the procedure. Inconsistent pH can change how substances react with each other. When hospitals run blood chemistry panels, keeping each solution stable ensures results accurately reflect what’s happening in the body. I’ve known clinical lab techs who swear by BES-Na-based buffers because they don’t react much with other substances in the test, sidestepping the headaches that some other chemicals bring.
Reliable in Protein and Enzyme Work
Protein chemistry depends heavily on the pH staying within a narrow band, especially when purifying enzymes or running electrophoresis. Small drifts in pH can unravel proteins, scramble results, or even waste valuable samples. BES-Na offers the right handshake: it holds a steady pH yet doesn’t interfere with most biochemical processes. Researchers working with DNA-based techniques, like PCR or sequencing, sometimes turn to BES-Na when the more common buffers fail to deliver clean, consistent outcomes.
The Chemistry in the Details
BES-Na works thanks to the sulfonic acid group — it resists picking up stray ions or reacting with other chemicals nearby. You won’t see big swings in performance from bottle to bottle if you source from a reputable supplier with tight controls. The sodium salt version dissolves well in water and makes it easy to mix up reliable solutions quickly. That may seem like a small convenience, but when time matters and every experiment counts, fast and predictable results are gold.
Looking to the Future: Safer and Better Science
Safety gets a quiet mention in many chemical discussions, but it’s central to why BES-Na claims a loyal following. Compared to some alternatives, it carries a lower risk of toxicity for delicate cells or live tissues. As science pushes forward to more sensitive assays and sophisticated therapeutic research, BES-Na offers a steady backbone without adding new layers of worry. There’s always room to make chemistry safer and simpler, and dependable ingredients like BES-Na pull their weight every day across research, diagnostics, and even next-generation therapies.
Why Care About How This Chemical Sits on the Shelf?
Tossing N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic Acid Sodium Salt, sometimes known as BES sodium salt, just anywhere on a bench or in a cabinet might seem fine at first glance. Anyone who’s spent time in a lab knows a jar on a shelf looks pretty harmless. I learned early on that sloppy storage not only wastes supplies but also risks research and even personal safety. Dry chemicals like BES sodium salt collect moisture from the air like a magnet. I once pulled a container out after months in the wrong spot and found a soft, sticky mess instead of free-flowing powder. It ruined weeks of planned experiments.
Main Conditions That Matter
Storing this kind of buffer keeps me on my toes because even a small slip-up can change the pH of a solution, spoil the compound, and send whole projects off track. The salt form remains stable in dry air, yet start letting it soak up water or heat and those helpful properties disappear. Keep containers well sealed at room temperature; don’t cramp them next to the water bath or leave them somewhere the sun reaches through the window. Any exposure to light, especially if the cap isn't tight, can lead to slow but steady breakdown.
Desiccators are a lifesaver if you have to work in a space with summer humidity or worse, leaks from a faulty air conditioner. Vacuum-sealing helps keep air out, so the powder stays crisp and measurable. In crowded labs, refrigerator space seems like a win, but I’ve seen frost and condensation fog up a jar the first day someone leaves it open in the cold. That moisture shortens shelf life and can lead to strange, unpredictable results in sensitive assays.
Watch Out for the Little Things
It’s tempting to grab a bottle off a shared rack, weigh it out, and shove it back with gloved hands. Cross-contamination from bits of other chemicals—chlorides, acids, and those sneaky flakes of salt—sneaks into the open jar. Taking a moment to clean hands and pouring off what’s needed into a secondary container brings long-term payback. Even a tiny bit of contamination drags purity down, and in biochemical applications, there’s no hiding a bad batch.
Security and Records in Chemical Storage
Work in larger labs taught me that clear labels and careful inventory help prevent both misuse and waste. In regulated settings, labeling with name, preparation date, and hazard info reduces the risk of someone grabbing the wrong bottle. If the stock sits longer than a year, periodic checks for changes in color, lumps, or separated crystals save much hassle. Good recordkeeping also stops staff from doubling up on unnecessary orders, saving limited research funds.
Solutions from Practice
Taking these lessons together, every researcher benefits from small habits: closing lids right after weighing, keeping substances dry, checking labels and expiry dates. Investing in basic storage—airtight containers, desiccant pouches, periodic checks—answers most concerns. Sharing these habits through short team meetings goes further than any rulebook or official guideline.
Simple approaches guard both the chemical and the research, making sure N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic Acid Sodium Salt does what it’s supposed to the next time someone reaches for it.
What is N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic Acid Sodium Salt?
N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic acid sodium salt, often called BES sodium salt, finds use in biological labs and research environments mostly as a buffer. So, it helps keep a steady pH in chemical and biological solutions. Most people outside the science fields won’t run across it in their regular lives, but it does play a big role in studies involving proteins, enzymes, and cell cultures.
Reading the Label: Is It Hazardous?
This compound generally ranks as a “low hazard” chemical. That means for most purposes, it doesn't threaten people the way classic lab dangers like concentrated acids, heavy metals, or organic solvents do. Even so, any substance with a complicated name like this can raise concern, and that’s fair. Safety needs attention, no matter the risk level on the label.
Most chemical safety data sheets show BES sodium salt as causing irritation in the eyes or on skin, but not much else. Swallowing it would bring some gastrointestinal distress—nausea or stomach pain, mostly. Comparison with kitchen salt or baking soda might help put it in context. Both those household products could also cause irritation in the wrong setting or at the wrong amount. Still, nobody wants to deal with a chemical burn.
Toxicity Data and Real-World Risks
Lab tests and animal studies reveal very little evidence of significant toxicity with BES sodium salt. No reports stand out about it causing cancer, birth defects, or serious illness in people or animals. In the wild, this compound won’t build up and threaten wildlife. It breaks down and travels through water supplies without damaging aquatic life. Proper disposal matters for any chemical, but trashing a buffer like this won’t poison the neighborhood water table.
I’ve spent time working in research settings, handling powders and buffer salts similar to BES sodium salt almost every day. Gloves always go on before opening any bottle. A dust mask or fume hood comes into play when the risk of airborne particles pops up. Some folks take shortcuts, but my hands remember the sting from carelessness. Even if a chemical ranks as “low hazard,” touching your eyes after handling any lab powder can ruin a day fast.
Safe Use and Solutions for Safer Labs
If you mix BES sodium salt into a solution or clean up any spills, plenty of water and a wipe-down keep things in line. Good ventilation makes life easier and lowers the odds of accidental inhalation. Most labs now run safety training with hands-on demonstrations, not just reading the fine print on a safety sheet. That helps drive home real practices: closed containers, clear labeling, and routine hand-washing after handling any lab material.
Many schools and companies now use digital safety logs and QR codes linking directly to the safety data sheet for every chemical in the lab. That shift gives instant answers when someone needs quick info on handling or emergency steps. Teachers also share simple stories about slip-ups—like eye-rubbing gone wrong, or a buffer salt spill—because real experiences stick much better than dry technical guidelines.
Balancing Usefulness and Caution
BES sodium salt gives modern science a reliable buffer for tricky experiments. Despite its science-heavy name, its actual risk stays pretty low. Respect for any chemical—even ones with a “safe” safety sheet—will always matter. Quick and simple steps with gloves, washing up, and clean workspaces make sure this compound, or anything else on the shelf, won’t cause trouble. That approach in the lab or at home with cleaning products pays off every time.
Understanding the Chemical's Nature
Working around chemicals like N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic acid sodium salt—commonly known in labs as BES sodium salt—means any slip can lead to problems down the line. Safe handling calls for common sense but hinges on good habits: gloves, goggles, and lab coats never feel optional, especially after years in the lab. This compound won’t eat through gloves like strong acids, though skin contact or inhalation can lead to irritation. Anyone who’s suffered a chemical splash to the skin or eyes can testify how quickly irritation takes over. Immediate washing with soap and copious water usually heads off lingering problems, but prevention stays easier than an emergency rinse.
Safe Storage and Daily Handling
Beakers of BES sodium salt belong under ventilation or in a fume hood—both for peace of mind and because powders spread easily. Sealing containers tightly keeps air humidity away and prevents clumping. Once, a poorly closed container clumped so badly the powder transformed into sticky lumps, forcing us to throw away what could have been used safely. Keeping the storage space dry and out of sunlight stretches shelf life and keeps the material easy to measure and transfer. Over the years, a habit developed: label every container with clear dates and full chemical names. Unlabeled or vaguely marked jars have landed many labs in trouble with audits or with confusion during hectic research.
Minimizing Exposure and Spills
Handling BES sodium salt means more than just safety gear—it means paying attention. The powder might not billow like flour, but any loose dust carries risk, even if that risk seems lower than for stronger toxins. Small spills on benchtops can be swept up with damp paper towels, then washed down with lots of water. Large spills call for dedicated spill kits—scooping up dry powder gently, to minimize breathing in the stuff.
Putting chemicals back in the right zones after use never feels like overkill; avoiding cross-contamination between different powders keeps everyone safe and experiments clean. At the end of a day, a wiped-down work area goes far toward preventing unexpected mixing or issues with future work.
Smart Disposal Practices
Even stable compounds like this one require thoughtful disposal. Pouring solutions or powders into a sink invites trouble for the water supply, as municipal systems aren’t built to filter out every lab chemical. I’ve seen enough near-misses to know how quickly a misplaced waste bottle can cause headaches—not just for researchers but for the whole building. Use of a properly labeled, compatible waste container prevents both accidents and anger from lab managers.
Following institutional or regional hazardous waste guidelines remains the only reliable way. Bottles go straight to the facility’s collection point, never mixed with incompatible substances. Too often, those rushing to finish for the day stack used containers in the wrong place or forget to log hazardous contents. Clear logs and double-checking labels ensure that disposal pros know exactly what they’re handling. Properly segregating and tracking waste isn’t only legal, it respects everyone downstream—maintenance crews, waste processors, and even the wider community.
Building a Culture of Responsibility
Training should happen regularly, hands-on, and with realistic scenarios—far more effective than just pamphlets. Watching a colleague ignore a spill or shortcut a disposal step under pressure has always triggered a reminder: individual actions ripple out into group safety. The simplest routines, backed by a culture that values attention to detail, do more to protect health and the environment than fancy safety posters.
Navigating Lab Realities and Industry Needs
Years in the lab have made one truth painfully clear: the difference between a good experiment and a failed one often lies in the details most folks gloss over. Purity specification for chemicals like N,N‑Bis(2‑hydroxyethyl)‑2‑aminoethanesulfonic Acid Sodium Salt is one of those details that can decide your entire outcome. This salt — known to many as BES-Na — plays a big role as a buffer in biological experiments. You trust it to keep your system stable, but trust only happens when you know exactly what’s in the bottle.
What’s Considered ‘Pure’?
Quality chemical suppliers usually list purity for BES-Na as ≥99%. That's not a magic number someone pulled out of thin air. Manufacturers run a combination of analytical tests:
- Loss on drying: Measures how much weight disappears if you heat the salt — a sign of water content or volatility you don’t want in your recipe.
- Heavy metals: Labs run scans for heavy metal traces, often setting the bar at less than 5 ppm for lead, mercury, and others. Even tiny contamination alters your results, sometimes in ways you won’t see until your project veers off-track.
- Chloride and sulfate content: Good batches of BES-Na keep chloride under 0.01% and sulfate under 0.05%. Chloride sneaks in from shortcuts during synthesis, and sulfates whisper that someone didn’t clean up after neutralizing or isolating the product.
- Assay by titration or HPLC: For BES-Na, a proper purity spec uses direct measurement, aiming for an assay between 99% and 101%, which covers the balance of accuracy and practicality in commercial production.
- Identity Test (IR/NMR): Anyone worth their pipette checks that what's on the label matches what's in the jar.
Why Does Purity Even Matter?
Every researcher who’s ever spent hours staring at a failed electrophoresis gel or an unexplained drop in cell viability knows that even the smallest impurity can tank your work. BES-Na as a buffer doesn’t do its job if it comes tainted with metal ions or unwanted side-products. That traces right back to the purity guaranteed by your supplier.
Beyond that, researchers doing protein isolation, diagnostic kit preparation, or gene editing can’t afford surprises. Impurities might get in the way, spark corrosion, or confuse downstream analysis. At best, your yield drops. At worst, you publish misleading data — a risk nobody needs.
Risks and Real-World Solutions
Smart labs go beyond trusting the spec sheet. Always ask for the latest Certificate of Analysis. Dig into the batch data: look for chromatography profiles or third-party testing reports. Don’t hesitate to run your own purity controls, especially if you push boundaries with sensitive downstream applications like drug development or high-throughput screening.
If budgets drive you toward lower-grade chemical grades, double up with validation steps. Cross-check pH stability, confirm with standard reference materials, and always label your stocks clearly. I’ve watched too many experiments waste weeks due to mislabeled or substandard buffer salts.
Key Takeaways for the Real World
A 99% spec for BES-Na isn’t just paperwork—it’s the foundation of reliability and reproducibility. There’s no shortcut past hands-on scrutiny and sharp attention to the details suppliers publish. In a research setting, where every step rides on trust in your reagents, purity isn’t an abstract goal—it’s ground-level reality.