N-Methyl-2-fluoroaniline: A Commentary on Its Development and Impact
Historical Development
People have chased after better building blocks for pharmaceuticals and specialty chemicals for over a century. The journey around aniline derivatives has walked hand in hand with the rise of synthetic organic chemistry in the late 19th and 20th centuries. N-Methyl-2-fluoroaniline, with its methyl group and a fluorine anchored onto the aromatic ring, sits as a product of chemists’ efforts to introduce functional groups that bring about useful properties. Fluorinated aromatics caught attention during the hunt for stable, bioactive molecules—pharmaceutical chemists saw in them a means of fine-tuning activity and boosting metabolic stability. It grew out of the practical needs of researchers who sought simple ways to build complexity into chemical structures, and its progress paralleled advances in fluorination techniques and methylation strategies which became routine by the late 20th century.
Product Overview
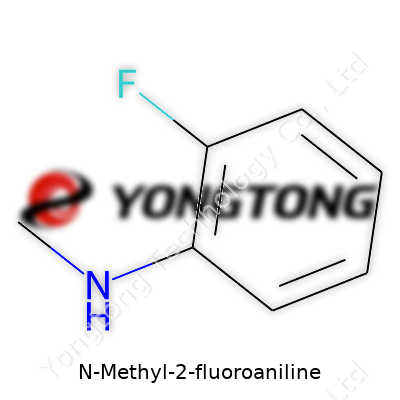
N-Methyl-2-fluoroaniline stands out as an aromatic amine, shaped by a methyl on the nitrogen and a fluorine attached at the ortho-position of the benzene ring. The purpose of adding both a methyl and a fluoro group, beyond simple curiosity, traces back to how these tweaks deliver major shifts in solubility, steric profile, and reactivity. Folks in R&D often reach for it when seeking to build molecules where electronic effects steer reactivity or tailor drug-like properties.
Physical & Chemical Properties
This compound brings a clear, sometimes pale yellow appearance with an aromatic odor, showing up as a liquid at room temperature. Boiling at around 187–189°C, it sports a melting point typically below zero. The aromatic ring, softened by the electron-donating methyl and electron-withdrawing fluorine, creates a push-pull effect that changes both acidity and electron density—a big deal for chemists chasing specific synthetic goals. Solubility in organic solvents (ether, chloroform, acetone, toluene) gives it chemical flexibility, and limited water solubility draws boundaries on its environmental mobility.
Technical Specifications & Labeling
Chemical companies stamp every container with CAS number 366-34-1, molecular formula C7H8FN, and a molecular weight near 125.14 g/mol. Labels bear hazard codes aligning with international standards, marking it as hazardous for skin, eyes, and sometimes for aquatic life. Users must wear gloves and goggles, backed up by storage signage that warns against open flames or prolonged exposure. In my experience, compliance with global GHS labeling not only keeps labs honest but also gives peace of mind, especially as regulations across regions have their nuances.
Preparation Method
The route to N-Methyl-2-fluoroaniline usually traces back to either direct methylation of 2-fluoroaniline or nucleophilic aromatic substitution reactions. Chemists commonly pull in methyl iodide or dimethyl sulfate under basic conditions, keeping a steady hand to manage exotherms and reduce byproducts. Many operations run batchwise with cooling jackets to control heat. Industrial setups often recycle solvents and use catalytic bases to cut down costs and reduce environmental load. Purification by distillation under reduced pressure or chromatography cleans up the product for downstream use.
Chemical Reactions & Modifications
N-Methyl-2-fluoroaniline acts as both a fine substrate and an intermediate. The nitrogen, now a secondary amine, partners with electrophiles for alkylations and acylations. The fluorine, resistant toward substitution due to strong C–F bonds, often endures tough conditions unless forced off by strong nucleophiles. The ring’s altered electronics steer the outcome of nitrations or halogenations, letting researchers direct transformations with higher precision. Chemists have built dyes, herbicides, and drug molecules starting from scaffolds like this, linking further aryl, heterocyclic, or alkyl units.
Synonyms & Product Names
You’ll find other names—N-methyl-o-fluoroaniline, 2-fluoro-N-methylaniline, MMAF—on catalogs and labels. Older synthesis reports sometimes call it N-methyl-2-fluorophenylamine. The differences usually come down to supplier tradition or regulatory context, and recognizing them sheds light on the product’s broad utility.
Safety & Operational Standards
Handling aromatic amines often reminds chemists that safety isn’t optional. N-Methyl-2-fluoroaniline, much like its cousins, can irritate skin and airways, pushing labs toward gloves, face shields, and effective ventilation. Proper training goes further than any label: everyone learns early on about safe weighing, avoiding inhalation, and spill management. Regulatory agencies in the US, EU, and APAC keep tight tabs on reporting, worker exposure, and effluent discharge. Waste management—sorbents for spills and high-temperature incineration for disposal—follows sharp protocols. Relying on standard operating procedures anchored in OSHA or REACH frameworks makes a vast difference in risk reduction.
Application Area
Chemists and manufacturers value N-Methyl-2-fluoroaniline for making agrochemicals, pharmaceuticals, and dyes. The methyl group gently increases lipophilicity, the fluorine tampers with metabolic attack—both features that help fine-tune drugs for better performance in the body. In agrochemicals, the compound often slips into active ingredients that need environmental persistence without heavy toxicity. The dye industry uses its strong ring system for azo compounds that resist fading and bleed. The broader story isn’t only about the molecule’s function but also the problem-solving that each application represents—a balancing act between reactivity, durability, and safety.
Research & Development
R&D groups have driven much of the progress by pushing N-Methyl-2-fluoroaniline’s chemistry into new territory. Structure–activity relationship studies in drug design lean on the methyl and fluoro effects to find optimized candidates. Medicinal chemistry teams use it for synthesizing kinase inhibitors and central nervous system drugs, trying to sidestep the usual pitfalls of metabolic degradation. Teams working on material science keep an eye on its electronic effects for crafting smarter polymers and sensors. The constant give-and-take between discovery and application nudges synthetic methods toward greener, safer, and more cost-effective choices.
Toxicity Research
Toxicologists have flagged aromatic amines as a group for close scrutiny, and N-Methyl-2-fluoroaniline is no exception. Short-term exposure irritates mucous membranes and skin; chronic tests in animal models often signal concerns about organ toxicity if handled carelessly. The limited biodegradability of fluorinated groups signals persistence in the environment, raising flags about aquatic toxicity. Regulators in the US and Europe keep a close watch on its environmental fate, prompting regular updates to workplace exposure guidelines and wastewater thresholds. My time working with environmental testing showed the importance of precise detection methods—GC-MS being a standard for tracking residues.
Future Prospects
Looking ahead, chemists expect N-Methyl-2-fluoroaniline to find broader use as the search for new pharmaceuticals, crop protectants, and advanced materials presses on. The future of its use seems tied to advances in green chemistry: safer reagents, less toxic byproducts, and better waste recovery systems. Regulatory shifts will probably push even harder for lifecycle management and full disclosure of environmental impacts. Academic and industrial partnerships can drive forward smarter synthesis, more thorough toxicology profiling, and even creative recycling paths for byproduct streams. The quest for safer, smarter chemicals has never let up, and compounds like N-Methyl-2-fluoroaniline play a big part in shaping that future.
Understanding the Building Blocks
Chemical structures shape what happens in the real world. Take N-Methyl-2-fluoroaniline, as an example. Its molecular formula is C7H8FN. Getting the formula right isn’t just about dotting i’s and crossing t’s—it brings clarity for every chemist, formulator, or researcher working with the substance. In practice, that formula reveals a lot. It points to a benzene ring carrying three modifications: a methyl group at the nitrogen, an amino group attached directly, and a fluorine atom on the second carbon of the ring.
Importance in Today’s Laboratories
I’ve seen firsthand how clarity about molecular structure saves both money and time. I remember one project where the smallest overlooked functional group turned a manageable synthesis into a multi-week tangle. Labels matter, especially in settings where a single atom tweak—like swapping a hydrogen for a fluorine—can change a compound’s uses, both in industry and research. The fluorine atom in 2-fluoroanilines often brings unexpected durability and distinctive reactivity, which influences everything from safety protocols to patent claims.
Fluoroanilines, and their derivatives such as N-Methyl-2-fluoroaniline, show up in dyes, pharmaceuticals, and materials research. The combination of methylation and fluorination shifts the way the molecule interacts with enzymes, cell membranes, or other chemicals. If you’re on the formulation or discovery end, understanding those facts means you avoid costly repeat experiments and wasted reagents.
Why Accuracy in Naming and Formulae Matters
Error rates rise in the lab or on the production floor when formulas go wrong. Several years ago, a trusted academic journal had to issue a correction on a family of substituted anilines. This correction forced dozens of research teams to revisit their own notebooks and publications. It reinforced the core tenet: trust in your building blocks or pay for it with repeat work.
Regulatory guides, too, use these formulae as linchpins. C7H8FN meets very different restrictions from, say, simple anilines due to its methyl group and the reactivity boost from fluorine. Downstream manufacturers rely on the clear assignment of these formulae to comply with chemical safety inventories worldwide. If an error slips by, penalties follow or shipments get stuck in customs. The impact ripples out to jobs, research grants, and business contracts.
Paths Toward Greater Consistency
To dodge mislabeling—whether on shipping manifests or in journal abstracts—training matters. Many younger scientists learn naming conventions in a hurry. Seasoned chemists double-check their work, sometimes out of hard-won habit. Digital validation tools help, but I’ve found that peer review and rigorous cross-checking stand above software in catching subtle errors. Guidance also comes from organizations like IUPAC, whose rules aren’t always simple but remain reliable touchstones in a shifting field.
In research and industry, precision isn’t a burden—it’s the groundwork for every safe, successful project. Next time you see a plain molecular formula like C7H8FN, remember the layers of trust and expertise built into those characters, earned by generations of practitioners, and critical to progress both in the lab and out in the world.
How Chemistry Shapes Our World
Small tweaks in a molecule can have a big effect on how it’s used. N-Methyl-2-fluoroaniline doesn't get headlines, but it touches more lives than you might think. Step into any research lab working on new medicines or advanced materials, and chances are someone is running a reaction that counts on building blocks like this one. As a person who has spent nights staring down stubborn lab glassware, I know just how important these little compounds can be.
Pharmaceutical Research Relies on Small Players
Think about drug development. Scientists look for new ways to tweak biological pathways, often starting with amine-containing chemicals. N-Methyl-2-fluoroaniline serves as a starting piece—chemists call it a “precursor”—when they want to make drug candidates. That fluorine atom isn’t just for show. It helps the molecule fit in different spots inside the body, which can affect how medicine works or how long it stays active. You won’t see N-Methyl-2-fluoroaniline on a pharmacy shelf, but without intermediates like this, many treatments for heart disease, infections, or cancer simply wouldn’t exist.
Agrochemical Innovation Counts on Smart Chemistry
The push for better, safer pesticides and weed killers doesn’t get any easier. Farmers and researchers want effective solutions but also less environmental impact. N-Methyl-2-fluoroaniline helps in making some of the “active ingredients” in newer agrochemicals. Adding fluorine atoms to molecules can make them work longer or break down in a way that cuts down on harmful leftovers. Growing up in a rural area, I saw how poorly designed chemicals could wipe out more than just pests. Careful design, starting with molecules like N-Methyl-2-fluoroaniline, helps avoid those mistakes.
Dyes and Advanced Materials Drive Demand
Walk through any textile dye lab, and pages of synthesis plans line the walls. N-Methyl-2-fluoroaniline finds its way into specialty dyes, including those used for technical fabrics or printing electronics. That slight change in the molecule—the fluorine swap—can give color compounds new properties, maybe boosting their ability to resist sunlight or cling to tough surfaces. Engineers working on organic electronics sometimes use related molecules to help move electric charges or to build new types of sensors. These aren’t uses you see advertised, but they shape products all around us.
Staying Safe, Reducing Environmental Impact
Anytime a chemical gets used widely, people start worrying about safety or what happens when it escapes into the air or water. N-Methyl-2-fluoroaniline can pose risks if handled without care; it can irritate the skin and eyes, and inhaling it raises health questions too. Over the years, I’ve seen research labs turn safety training into an everyday routine, checking ventilation and reviewing safety documents for every project. Factories use closed systems and strict disposal practices, but small-scale users still face challenges, especially where safety culture lags or regulations aren’t strong.
Cleaner reactions can cut down on waste. There’s a strong push now to use greener solvents or invent ways to react and recycle chemicals without making a mess. For N-Methyl-2-fluoroaniline, these changes could come in the form of new catalytic methods, less harsh reaction conditions, or better containment tech. Solutions like these take effort, but when you see them put to work, everyone—workers, neighbors, and the planet—benefits.
Understanding the Risks Upfront
Every time I walk into a lab, the very first thing on my mind is what I’m handling and how it might get me in trouble if I’m not careful. N-Methyl-2-fluoroaniline isn’t something to shrug off. Weigh in what the Safety Data Sheet explains: This chemical can cause skin and eye irritation, it’s harmful if inhaled or swallowed, and the fumes or contact can trigger allergic reactions. Folks use it for research or in the synthesis of specialty chemicals, but that doesn’t mean we treat it any more casually than anything else toxic on the bench.
Planning Safe Handling
I always look at the handling steps as common sense. Leaving safety to chance never made much sense. Before opening a bottle, I set up in a well-ventilated fume hood—no exceptions. One tiny spill or a waft of vapor in a tight room could cause headaches, sore throats, or worse for anyone around. Personal experience taught me the hard way that regular gloves might not stand up to some solvents. For N-Methyl-2-fluoroaniline, I stick with chemical-resistant nitrile gloves, splash goggles, and a sturdy lab coat. If something splashes, I don’t want it anywhere on me.
There’s a story from grad school that comes to mind: a friend got distracted while transferring a similar aniline compound and missed a drop on the bench. It took only ten minutes for him to start feeling light-headed, just from a little airborne vapor. That memory makes me double-check the fit on my mask whenever something volatile is involved. Appropriate respiratory protection hangs above every chemical workspace for a reason, and we can’t afford to get lazy about it.
Containment and Storage
I’ve seen folks toss caution aside, leaving reactive or volatile chemicals on an open shelf. With N-Methyl-2-fluoroaniline, those risks just aren’t worth it. I always store anything volatile or reactive in tightly sealed bottles, tucked away in a cool, well-marked cabinet, far from oxidizers and acids. Some spills can release toxic vapors or even catch fire, and nobody wants to clean that up—or deal with an emergency in the middle of a busy workday. I keep a dedicated spill kit nearby, not just for company rules but for peace of mind.
Good Practices and Emergency Readiness
Good habits build safety. I’ve always trained team members to label every single container, even if you think you’ll come right back to it. Wiping down surfaces after handling these sorts of chemicals seems simple, but I’ve seen labs get shut down just because no one cleaned up a few drops after a late night. I also run through the emergency shower and eyewash routine with every new hire. It’s awkward, but if something goes wrong, seconds matter.
From personal experience, I know many accidents come from people skipping simple checks—like reading labels or ignoring protective gear for a “quick” task. Folks sometimes forget that chemicals like this don’t always give a warning before causing harm. Safety comes down to people caring enough to do the right thing every time.
Solutions: Building a Culture of Respect
Supporting each other is the best insurance we have. I’ve seen labs thrive when chemists look out for each other, share stories about near-misses, and keep everyone honest about good safety habits. It helps to post clear protocols and keep tools like spill kits, goggles, and gloves visible and stocked. Prepping for worst-case scenarios—like practicing spill response—never wasted anyone’s time. Leadership matters, but so does each person’s willingness to slow down and follow the basics.
At the end of the day, handling N-Methyl-2-fluoroaniline safely isn’t about fear—it’s about respect for your work, your health, and your team. Science doesn’t wait for mistakes, but careful people catch them before they happen.
Real-world Stakes in Pure Chemistry
Anyone who’s worked with specialty chemicals knows the difference a tiny impurity can make. With N-Methyl-2-fluoroaniline, the tolerance for error stays slim. In my own research years, lab supervisors hammered into us: check your Certificate of Analysis, don’t take purity for granted. Fresh out of university, I saw firsthand how a 99.5% bottle kept a synthesis clean, while a “just okay” technical grade stalled reactions or filled your product with noise. It was a sharp lesson—purity can save or waste weeks.
Defining Purity in Practice
Reputable suppliers state specifications right on the label. For N-Methyl-2-fluoroaniline, the expected purity usually falls at or above 98%, with HPLC or GC data to prove it. Advanced users—pharma labs, electronic materials teams—push for 99% or more. Lower grades invite unpredictable results in both the lab and the plant. Impurities show up differently—sometimes as water, sometimes as leftover aniline, sometimes as trace ethers or halides. Those can kick off side reactions, drop yields, or worse, drift into a patient’s medicine.
Contamination Can Hurt Downstream
My first contract job put me on a pesticide synthesis line. We lost thousands one month because a solvent supplier cut corners. The impurity profile shifted, our purified product’s color changed, registration failed. That woke me up to the bigger picture. Slight shifts in purity—never mind visible particles or odd colors—can halt everything from pilot studies to regulatory submissions.
What Drives Purity Level Demands?
People sometimes ask, “Why bother with such high specs?”, usually after seeing the price. Drug development illustrates the answer: the FDA and EMA both scrutinize raw material purity. A single unknown impurity or variation between lots means extra paperwork, more tests, and setbacks. In electronics, even a faint presence of ionic or metallic impurities creeps into end-use reliability. To stay ahead, suppliers put out data for every batch, and buyers double-check spectral signatures right after delivery.
Verifying Claims, Keeping Trust
I’ve seen experienced chemists run a quick GC on incoming shipments, even from trusted sources. That bit of paranoia keeps projects on track and builds trust with quality teams. If numbers look off, buyers call out the supplier right away. Anything under 98% without a note gets rejected. My own lab once sent three liters straight back and logged a procurement investigation. A good supplier shares batch data, answers questions, and regularly updates their analytical techniques.
Beyond One-Size-Fits-All Approaches
Some applications tolerate minor impurities, but research groups and regulated industries stand by the strictest specs. Rather than relying on blanket percentages, they list out which impurities shouldn’t be present at all, and which should stay below a certain part-per-million. This level of detail reassures the final buyer, whether it’s a physicist building OLED displays or a biologist testing a new probe.
Building Strong Supply Chains
Strong relationships between users and suppliers build the backbone of high-quality chemical supply. In my experience, opening doors for dialogue and quality audits leads to fewer surprises. Distributors who back their specs with transparent documentation win loyalty—nobody wants to debate over mystery peaks during an inspection. For those staking research money or patient safety, agreeing on clear, proven, and achievable purity specs for N-Methyl-2-fluoroaniline isn’t just paperwork—it’s common sense.
Understanding the Risks
N-Methyl-2-fluoroaniline isn’t just any chemical you poke at in a lab. The compound comes with a sharp set of risks. Toxic fumes, skin irritation, potential for fire — you get a real lesson in danger if you underestimate it. It surprises me how quickly something that looks harmless can turn into a serious emergency with a single careless move. From news stories about chemical leaks to the rare fire in an academic lab, the lessons stay the same: respect the compound, follow the rules, or deal with the consequences.
Conditions That Keep N-Methyl-2-fluoroaniline Safe
Keeping this chemical in check starts with temperature. Heat and sunlight accelerate decomposition and release hazardous fumes, so a cool, shaded storeroom works best. I’ve visited industrial settings that use temperature-controlled units holding steady around 2-8°C. These setups save a lot of worry, especially since even small lapses can create serious risks for workers or nearby communities.
Humidity introduces its own problems. Moisture eats away at containers faster, raising the chance of leaks. So, it makes sense to store this aniline in a dry zone, with good ventilation and reliable spill containment. Metal shelves away from water sources—simple measures like these can prevent a lot of headaches.
Container Choices Matter
Glass and certain high-density plastics hold up the best. Metal reacts with N-Methyl-2-fluoroaniline and creates purity concerns, plus some ugly chemical by-products. Containers must seal tightly to trap both liquid and vapors. I remember touring a facility where cheap caps failed during hot weather. The resulting vapors set off alarms and sent people scrambling. It gets expensive and dangerous quickly—sturdy, chemical-resistant seals pay for themselves in peace of mind.
Clear labeling with hazard symbols, batch numbers, and emergency contact details help staff and responders work smarter in an emergency. Labels that peel or fade cause confusion, so waterproof markers or printed plastic labels get the job done right.
Transportation: No Shortcuts Allowed
Moving N-Methyl-2-fluoroaniline isn’t just a drive across town. Deliveries follow strict international and local rules (like the UN’s Recommendations on the Transport of Dangerous Goods). Officials inspect loads to see if proper paperwork and packaging are in place—not an area where shortcuts pay off. Specialized chemical transport vehicles feature containment pans and racks designed for hazardous freight. I’ve seen cases where using ordinary vans led to leaks, angry neighbors, and cleanup bills that dwarf the cost of doing things correctly the first time.
Drivers receive hazmat training to tackle spills or evacuation orders. They carry chemical-resistant personal protective equipment, fire extinguishers, and spill kits. One driver shared how practice drills helped him react fast to a small leak, keeping everyone out of harm's way and protecting the environment.
What Works, and What Needs Work
Storing and moving N-Methyl-2-fluoroaniline relies on discipline and investment. Routine inspections spot leaks and decay before disaster strikes. Automation and sensors for temperature, humidity, and vapor levels give nearly real-time warnings. Staff can act before people or property take a hit.
Some small-scale operations skimp on equipment or training, gambling that nothing goes wrong. Joining industry safety groups and learning from more experienced peers offers a huge boost. Public agencies and suppliers publish guidelines, but success often comes from learning what works on the ground, sharing stories, and keeping safety at the top of the list. It’s everyone’s responsibility—whether you mix, store, ship, or simply work nearby.