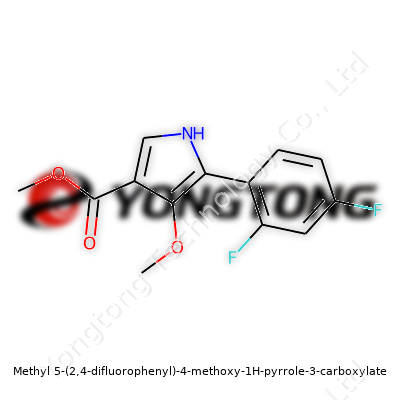
Methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate: Insight and Outlook
Historical Development
Organic chemistry rolls forward because new molecules open up new possibilities. Looking back, methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate belongs to a lineage of pyrrole-based compounds that keep making an impact in both the research lab and the world at large. Pyrrole frameworks first caught attention in the late 19th century, often drawing interest from chemists because of their presence in complex natural products like heme and chlorophyll. As research pressed on through the 20th century, fluorine incorporation—tricky but rewarding—grew more common, prized for its ability to change a molecule's electronic qualities, stability, and biological activity. In the past few decades, the combination of fluorinated aromatics and heterocyclic scaffolds has become standard for exploring drug discovery and material science, pushing molecules like methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate into the limelight. Seeing how rapidly this field has evolved, it’s clear the history of this compound follows a much bigger pattern: chemists remix familiar structures to chase new functions, often spurred by better synthetic methods and ever-rising curiosity about what these molecules can do.
Product Overview
Methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate carries a complex name, but at its heart, it’s a pyrrole ring sporting a couple of serious modifications: two fluorines on the phenyl group, a methoxy on the ring, and a methyl ester hanging off the carboxyl. This structure puts it at the intersection of several important trends in modern chemistry. Fluorination often steps up a molecule’s performance in pharmaceuticals, making it more resistant to metabolism and sometimes improving how it binds in the body. The methoxy group can tweak a molecule’s solubility and electronics. The carboxylate ester acts as a handle for researchers looking to tweak the molecule further, or tune its solubility. That collection of features makes this compound more than a simple building block—it turns it into a springboard for invention.
Physical & Chemical Properties
People who’ve worked with fluorinated aromatics and pyrroles know you don’t always get a predictable set of properties. This compound generally comes as a pale solid, not volatile or odorous. In the flask, its density and crystallinity can vary based on how precisely it's synthesized and purified. The presence of two fluorine atoms toughens up the structural backbone, offering a degree of chemical robustness when compared to plain pyrrole or phenyl analogs. The methoxy group tends to nudge the molecule’s solubility upward in common organic solvents. The methyl ester keeps things manageable, giving you a compound that handles well on the bench and offers a reasonable melting point for handling or storage. Chemists need to stay alert for sensitivity to moisture or strong acids and bases, since the ester can hydrolyze and the pyrrole ring could get messed up in harsh conditions.
Technical Specifications & Labeling
Working in a lab, nobody wants ambiguous labeling. This compound shows up with a CAS Registry Number, batch-specific purity data (often exceeding 95%), and typically boasts an HPLC or NMR accompanying the package to back up identity. Labs pay attention to storage temperature—usually cool, dry conditions—and clear routes for chemical traceability. Detailed labeling covers the IUPAC name, molecular formula, formula weight, and details on impurities. If purchased from reputable suppliers, the specification sheet highlights the spectral characteristics, melting range, and safety codes matched to international standards. All this data matters for any downstream work in research or manufacturing, since a single impurity can throw off results or even cause harm.
Preparation Method
Synthesis still feels like a mix of art and rules, especially for decorated pyrroles. Typically, the process starts with the functionalized phenyl partner—here, a 2,4-difluorobenzaldehyde or a related acid chloride. Through a Paal-Knorr or Knorr-type pyrrole synthesis, these precursors couple with an amine and a β-ketoester variant, laying down the skeleton. Selective alkylation with methylating agents delivers the methoxy group. To get the methyl ester in just the right spot, a controlled esterification—normally under acidic or basic conditions—finishes the job. The multi-step process often calls for purification by column chromatography, usually silica gel, to strip away side products. Watching a well-run synthesis in action highlights the precision needed, from controlling temperature to picking the right solvents. These decisions stack up, ultimately affecting how reproducible and scalable the process proves in practice.
Chemical Reactions & Modifications
This molecule stands ready for modification, and that’s what draws so many researchers. The methyl ester can hydrolyze to provide a carboxylic acid for coupling reactions. The methoxy substituent welcomes deprotection in orthogonal strategies or even further functionalization with electrophiles. The aromatic fluorines resist substitution except under extreme or highly specialized conditions, preserving the desired stability in biological environments or advanced material processes. Chemists running cross-couplings—such as Suzuki or Buchwald-Hartwig reactions—sometimes harness the aromatic side to install new groups, expanding options for drug discovery or material design. In the right context, selective oxidation or reduction can unlock unique derivatives, putting this compound at the crossroads of many synthetic routes.
Synonyms & Product Names
Names for this compound can stretch out or collapse, depending on use and region. You’ll see “methyl 5-(2,4-difluorophenyl)-4-methoxy-pyrrole-3-carboxylate” on research catalogues, sometimes condensed to “Difluorophenyl methoxy methyl pyrrole carboxylate.” In global chemical registries and patent documents, identifiers like the CAS number tie back to this structure, offering a universal common ground. Occasionally, research teams working on analogs assign their own internal codes or shorthand, which show up in academic papers or presentations.
Safety & Operational Standards
Working with small organics, especially ones with fluorine, comes with responsibility. Fluorinated aromatics can pose toxicity risks at scale, so labs rely on gloves, eye protection, and solid ventilation. Material Safety Data Sheets (MSDS) spell out known hazards—irritation to skin, eyes, or lungs, plus recommendations for spills or accidental exposure. Even in custom synthesis, safe handling protocols mean clear instructions for waste disposal, particularly because fluorinated waste streams sometimes defy easy treatment and need specialized incineration. Labs document every step from procurement to storage, including secondary containment and proper signage. Emergency procedures backstop the whole operation, because no shortcut beats a team prepared for chemical surprises.
Application Area
Anticipation runs high for what compounds like this can do past the beaker. Lead optimization in pharmaceutical chemistry draws on pyrrole scaffolds for both small-molecule drugs and diagnostic agents. Developers count on the fluorinated phenyl group to improve receptor selectivity or wait time in the body. Analytical chemists see value in its robust ring structure and substitution, useful as an intermediate for sensors or probes. Material science teams hunt for molecules that combine thermal stability, solubility tuning, and compatibility with organic electronics—here, the ester and methoxy play key roles. In agricultural chemistry, structural relatives of this compound serve as molecular templates for new fungicides and crop-protectants. Seeing diverse applications encourages further testing, as researchers rarely stop at the first promising result and push hard to expand a molecule’s utility.
Research & Development
R&D teams keep their notebooks open for molecules that tick multiple boxes: synthetic accessibility, stability, and the ability to tune physical properties. Research on methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate often means exploring structure-activity relationships (SAR), mapping which parts drive bioactivity or physicochemical traits. Computational chemistry now steps in before the first reaction—predicting binding affinities, simulating toxicological risk, or ranking modifications. High-throughput experimentation speeds up synthesis and screening, so teams can cycle through dozens of analogs. Collaboration with biology and engineering teams grows especially important for moving a compound from the bench to in vivo models or device integration, bridging theory with real-world test results.
Toxicity Research
Chemists and toxicologists never assume a new fluorinated pyrrole will sail through safety screens. Early-stage research delves into acute and chronic toxicity: in vitro cell assays map basic cytotoxicity, embryo models screen for developmental risks, and higher-level testing in animals traces absorption, distribution, metabolism, and excretion (ADME). Reports for pyrrole derivatives suggest watchfulness—liver metabolites sometimes intro trouble, and fluorinated aromatics linger in tissues longer than simpler cousins. Environmental scientists look downstream as well, tracking persistence in wastewater or soil. With regulators pushing for safer chemicals and transparent data, comprehensive profiling gets baked into the R&D pipeline.
Future Prospects
Predicting the next big leap in synthetic molecules means watching both the research that’s published and the conversations happening in the lab. As personalized medicine grows and materials science aims for smarter, tougher components, methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate stands out as a flexible workhorse. Its mix of tunable groups makes it a springboard for analog discovery, especially in the hunt for new pharmaceuticals or next-generation coatings. Advances in synthetic techniques—such as greener fluorination, automation, or machine-learning-guided synthesis—promise higher efficiency and safer processes. As regulatory standards evolve, especially around environmental impact and human health, the spotlight will keep shifting toward molecules that deliver benefits without outpacing our capacity to manage risk or recycle spent materials. Working chemists see not just another synthetic milestone but a step forward in designing smarter, more responsible, and more versatile molecules for tomorrow’s challenges.
Why This Molecule Even Matters
In the modern lab, chemicals like Methyl 5-(2,4-difluorophenyl)-4-methoxy-1H-pyrrole-3-carboxylate show up for one simple reason: chemists want efficient tools when tackling drug discovery. Structures like this come with specific traits—two well-placed fluorines, a methoxy group, and a pyrrole backbone. Together, these features offer an edge during the early activity screens and structural optimization of new pharmaceutical candidates.
Building Better Medicines
Medicinal chemists find value in this molecule’s design. Tweaking small pieces of a drug molecule can change everything: how it behaves in the body, how it interacts with proteins, even how resistant it is to being broken down by enzymes. The 2,4-difluorophenyl ring delivers more than a slick-sounding name—these fluorine atoms can help the core resist metabolic breakdown, turning a weak early hit into something stronger and more stable.
Drug design relies on these sorts of improvements. With resistance to digestion by certain enzymes, fluoro-containing fragments like this often last longer in the bloodstream. The methoxy group gives another control lever; scientists use it to fine-tune how well the molecule dissolves and how it latches onto biological targets. When chemists venture into the unknown, small tweaks make a huge difference—a lesson reinforced every time a new molecule goes through animal studies.
Library Synthesis: Casting a Wide Net
Ask anyone in early-stage pharma about how molecular libraries are built, and you’ll hear about scaffolds like this one. The pyrrole piece can serve as a starting block to attach new groups, test against multiple disease models, and explore structure-activity relationships. Essentially, it’s like having a modular base for constructing dozens or hundreds of slightly different drug candidates in search of one or two “hits”.
Researchers combine these building blocks in parallel synthesis to quickly fill out their screening collections. It speeds up the old “one molecule at a time” routine. As more companies move to automated chemistry and high-throughput screening, molecules like this carve out a place on the shelf, ready to jump into the next round of assays.
Chemical Innovation: More than Just a Building Block
It’s easy to underestimate the role of a single synthetic intermediate. Coming from a background in organic synthesis, I see this in how a specific group of atoms—once hard to prepare—now features in multiple research projects. Access to stable, pure intermediates like this has created more creative space for chemists. For example, a fluorinated ring or pyrrole scaffold that once took weeks to get can now arrive at the bench in a matter of days through specialty suppliers or custom synthesis shops.
Applying new chemistry to these building blocks leads to patent activity. Intellectual property value in pharma isn’t just about finished drugs—it starts much earlier, protecting the novel intermediates and pathways developed along the way. A unique core like this one might underpin not just a drug candidate but also a safer agricultural compound or an industrial additive.
Pushing Drug Design Forward
What stands out most about compounds in this class is versatility. For those working at the intersection of chemistry and biology, tools that let you adjust drug properties and speed up early testing can make a difference. When the right group of atoms lands in your hands, the path to a safer or more effective drug shortens. Inside the research trenches, that’s what matters most.
Looking Beyond the Label
Standing in a lab or a production facility, a simple question like “What is the chemical purity and grade of this product?” can shape the outcome of a whole project. Many treat it as a technical formality, but purity ties directly to safety, performance, and trust. If you have ever worked in a setting where a small impurity wrecked weeks of effort, you know not all chemicals are created equal.
Why Purity Shapes Results
Pure chemicals cut down risk. In labs, impure reagents give off weird colors, low yields, or sneaky results that burn through a research budget fast. In manufacturing, one off-spec raw material can shut down a production line. In my own experience, I have seen batches of product returned because a supplier switched from lab grade to technical grade without warning. Far from a “paper” issue, this cost real money and led to tough conversations with both customers and team members. Chemical impurities are not just numbers; they can introduce toxins, react unpredictably, or compromise safety. For pharmaceuticals, even tiny contaminants trigger recalls or worse, legal action. In electronics, trace minerals in the wrong place break delicate systems.
Grades Mean More Than Fancy Terminology
Chemical labels often list grades like reagent, ACS, USP, or technical. These aren’t meaningless. Reagent and ACS grades promise high purity for science, and most laboratories stick to them for analysis. USP, NF, and food-grade serve health and safety standards so strict that regulators inspect them constantly. Technical grade sounds good until you realize it accepts far wider impurity levels. I once worked with two bags of the “same” chemical: one from a specialty supplier marked ACS, one meant for industrial use. The ACS material sailed through critical tests while the cheaper industrial batch nearly caused a project delay.
Questions to Ask Suppliers
If you purchase or approve chemicals, blind trust is not an option. Suppliers should give certificates of analysis (CoA) for each batch, showing real data instead of marketing claims. Some try to hide behind broad ranges or out-of-date safety sheets. I have found that suppliers who can’t answer basic questions about their grades or make excuses over paperwork rarely deliver consistent quality.
Verifying What Really Arrives
Even with solid documentation, it pays to double-check. Spot checks using spectrometers, chromatography, or titrations can expose mislabeling or contamination. This is not overkill—one infamous case involved a mislabeled solvent that set off an explosion in a startup’s pilot lab. The cause turned out to be a missed impurity listed in fine print. Responsible labs rarely take material on faith alone.
Tracing the Impact on the End User
Products go far beyond labs and factories. Water treatment chemicals touch entire cities; pharmaceutical ingredients land in hospitals and pharmacies. Impurities in these products affect public health, legal liability, and even brand reputation. Bad press from a recall or failed audit can follow a company for years. I have seen teams spend weeks tracing one source of contamination, only to wish they had enforced tougher grade checks sooner.
Fixing the Problem Starts With Discipline
Clear internal guidelines keep mistakes in check. Set minimum purity requirements for every stage—and stick to them, no matter the cost savings on paper. Keep paperwork organized, demand details from suppliers, and train staff to spot red flags. It pays off every time a project launches on schedule without scrambling to explain impurities or rerun batches. Above all, treating purity as more than a technicality means fewer surprises, safer outcomes, and real respect for the people who rely on what we produce.
Real Impacts on Everyday Life
My grandmother used to keep apples in a cool, dark room off the kitchen. She didn’t use a fancy fridge or spend hours reading storage guides. Still, the apples lasted through winter. I learned early on that how you store things really matters—not just for taste, but for safety and money. Mistakes in storage can lead to spoiled food, wasted medicine, even ruined paint.
Why Storage Isn’t Just Following Rules
Some folks assume any shelf will do. Finished a shopping trip, toss everything in the pantry or fridge, and move on. But not all products respond the same way to light, air, heat, or moisture. Potatoes sprout in sunlight. Bread molds if left in plastic in a warm room. Aspirin in my medicine cabinet turned into powder long before the expiration date—humidity snuck in every time I showered.
Key Principles Backed by Science
Temperature swings speed up chemical reactions. Heat breaks down food nutrients and medicine potency. The FDA points out that most medicines stay stable around 20 to 25°C (68–77°F), away from steamy bathrooms or sunny windowsills. The CDC stresses that fridges should stay below 4°C (40°F) to slow microbial growth. I’ve seen milk go bad within hours from a faulty fridge, and it’s a rude awakening.
Humidity matters too. Dry cereal goes soggy if left out. On the farm where I grew up, grain silos had to stay bone dry. Grain at just a few percentage points above safe moisture would heat up, spoil, even combust. Bacteria and fungi love damp, warm environments.
Label Information Isn’t a Suggestion
Food, medicine, cleaners—labels list “store in a cool, dry place” or “refrigerate after opening” for a reason. Shelf life drops fast outside these ranges. Vitamins lose potency. Crisp vegetables become limp. Meat, when left too warm, can send people to the ER. In 2021, over 100,000 Americans landed in the hospital from foodborne illness, according to the CDC.
Common Sense Meets Modern Life
Few of us use root cellars or barns anymore, so we rely on machines that can break. Simple habits keep us ahead. Check fridge and freezer temperatures with a thermometer, rather than trusting the dial. Rotate your stock. Toss out forgotten leftovers before they become science experiments. Read storage notes on labels, and if in doubt, ask a pharmacist, a grocer, or look up manufacturer recommendations.
Technology Helps—But So Do Small Tweaks
For vaccines during a heat wave, clinics use backup generators. At home, a power outage means some food can move to coolers with ice. Medicines like insulin have specific storage needs—your pharmacist can provide insulated pouches for travel. Keeping humidity low is as easy as dropping desiccant packets in dry food staples and using tightly sealed containers.
Safe storage isn’t just about following lists or trends. It protects our health, our wallets, and sometimes even our lives. Learning a few basics, and checking our habits, makes a world of difference.
One Size Hardly Fits All
Over the years, I’ve found that the choice of package size for any chemical or compound matters a lot more than most people notice. In lab work, big drums look impressive, but in tight quarters, smaller bottles get top marks. A chemistry student once told me he needed just a handful of grams for a test, but the supplier only sold it in 25-kilogram bags. Storing that much in a small lab didn’t make sense, and nobody wanted to waste the rest. Multiply this problem across hundreds of chemicals, and storage rooms turn into awkward obstacle courses.
Businesses See the Same Struggle
Outside of academic labs, companies get squeezed by the same issue, just at a different scale. Think about small businesses launching a new product. They’re not ready to commit to truckloads of raw materials. If a supplier only offers giant tubs, costs skyrocket and leftovers gather dust. Meanwhile, big pharmaceutical plants might crave truckloads each week. Sudden jumps in minimum order sizes can slow things down or push up prices, harming small players most.
Real Impact: Money, Safety, and Waste
Misaligned package sizes often translate to wasted money. Small buyers pay high amounts for shipping and struggle to recoup costs when they’re stuck with too much surplus. I’ve seen labs try to donate spare reagents, but rules and shelf lives get in the way. In some sectors, like schools, tight budgets mean every gram thrown out hurts. Local hazardous waste disposal can cost hundreds for just a single container, so mismatched sizes quickly become a budget headache.
Safety takes a hit as well. Large packages, once opened, don’t stay fresh forever. Air or moisture creeps in after each scoop, degrading the quality or creating dangerous byproducts. Powdered substances, for instance, clump up or react over time. Researchers I know have stories about long-forgotten drums building up pressure or breaking down. Smaller vials might cost more per gram, but they actually keep people safer by limiting exposure and making stock easier to track.
Flexible Sizing: A Better Way
Some suppliers already offer flexible ordering. They list everything from tiny 10-milliliter ampoules to big bulk bags. Custom packaging sometimes gets offered, especially if you call and ask. Supply chain upgrades are making this process smoother. Digital inventory lets suppliers handle requests for oddball sizes, and companies picking up on this trend get repeat buyers who appreciate a little flexibility.
It’s also a place where government and education buyers have pushed for better service. Joint purchasing programs pool multiple groups’ orders, so suppliers agree to break up bulk shipments into manageable sizes. It all adds up to less waste, safer storage, and more affordable costs for groups that sometimes get overlooked by big players in the field.
The Path Forward
Fixing the mismatch in package options will take open lines between suppliers and buyers. Businesses can start by tracking their average stock turnover and telling suppliers what actually works for them in real-world settings. Suppliers with transparent catalogs and clear communication stand out. Regulators can chime in by supporting safe resale, donation, or return options for leftover compounds.
The chemistry industry keeps moving forward, but simple changes like smart packaging let more people take part, save money, and keep dangerous goods from gathering dust. It comes down to respecting how real people use these materials every day, not just how factories move them by the ton.
Why Chemical Handling Matters More Than Ever
Walking into a lab or an industrial site, most folks notice that tang of something sharp in the air. The right way to handle chemicals separates a safe day from an incident nobody wants. Years back, I watched a co-worker ignore a splash guard just to pour faster—he regretted it all week, because even small shortcuts can turn a basic task into a hospital visit. Chemical safety isn’t just a checklist; it’s about coming home in one piece.
Looking at Real Hazards
Everyone talks about wearing gloves, but it’s not only about tossing on whatever’s nearby. Each chemical tells its own story—some eat through latex in minutes, others evaporate so quickly they sneak right into your lungs. Splash goggles aren’t only for the dramatic spills; fumes attack eyes quietly. Chemical burns can look like nothing until the pain shows up hours later. Breathing in dust or vapor may seem harmless, but years of exposure add up. No lab coat or apron stays clean just by luck; serious accidents usually start with the “easy” tasks.
For example, hydrofluoric acid doesn’t just sting skin. It moves past pain and chews straight to your bones. Stories like this used to feel like exaggerations until people showed up missing work, nursing wounds from basic mistakes. The consequences of one wrong move stick around far longer than a missed deadline.
Learning from Mistakes
Too many beginners see safety instructions as background noise. One of my old mentors always said: “If you’re guessing, you’re gambling with more than your shift.” Simple steps make the difference—read the Safety Data Sheet each time a new bottle drops in. Pay attention to those fine-print warnings about heat, sunlight, water, or pressure. Mixing chemicals without thinking through what might gas off or explode isn’t just careless; it’s playing with risk for no reward.
Good habits come from facing the reality of what you’re handling. Flushing eyes immediately after a splash gives a fighting chance to save your sight. Locks and labels on storage cabinets save folks from grabbing the wrong thing in a hurry. Ventilation does more than clear out strong smells; it knocks dangerous vapors down to safe levels where lungs don’t pay the price. It doesn’t matter how many years someone’s worked around chemicals—if complacency creeps in, accidents follow close behind.
Ideas That Lower Risk
Teams that talk about near-misses learn faster than teams that hide mistakes. Eye wash stations and showers need regular checks, not just a sign taped up nearby. Keeping clean-up gear within reach makes the whole workplace safer for everyone. Spills cleaned up with the right absorbent stop slips and stop reactions from spreading. Wearing a lab coat and goggles doesn’t make anyone invulnerable, but they stack the odds in your favor every time.
Training sticks best when people run practice drills. Knowing exactly where to go and what to grab saves minutes when they matter most. No one likes pausing their day for safety talks, but the people who speak up and double-check end up preventing tragedy, not just inconvenience. Beyond rules, chemical safety comes down to remembering why each step protects real people, and how one small effort can keep close calls off tomorrow’s report.