Lithium Hexafluorophosphate: An Insightful Look at the Cornerstone of Lithium-Ion Batteries
Historical Development
Back in the late 1970s and early 1980s, engineers and chemists were puzzling over what would make rechargeable batteries both powerful and reliable. The demand for portable devices pushed the search past standard chemistries. The embrace of lithium chemistry changed the game, but this required a stable and conductive electrolyte. By the 1990s, lithium hexafluorophosphate (LiPF₆) had been singled out by researchers as the right answer. What seemed like a technical detail shaped the trajectory of mobile phones, laptops, and electric vehicles. LiPF₆ gave batteries a real boost in safety, voltage stability, and conductivity compared to salts like lithium perchlorate, which came with plenty of headaches over safety and shelf life. Even now, the rush to improve batteries ties right back to the unique properties first studied decades ago.
Product Overview
Crystal-clear and sharp-edged, commercial lithium hexafluorophosphate usually appears as a fine white powder. The demand coming out of battery factories worldwide has grown year over year, tying this one compound to global trends in electronics and clean energy. Producers don’t just churn it out; quality benchmarks—whether for moisture content or impurity thresholds—dictate whether the material can actually make it into a high-performance battery. Some names people use on product sheets include LPF6, Lithium phosphate hexafluoride, and the simple “battery-grade LiPF6.” Makers pull out all the stops to keep this material dry and contaminant-free, given its critical job inside each battery cell.
Physical & Chemical Properties
There’s a practical reason labs keep LiPF₆ inside airtight containers. It shows up as white crystalline powder, but open it to even a sniff of moisture, and it reacts rapidly, producing hydrofluoric acid (HF). HF, by the way, can corrode glass and eat through tissue, so this isn’t a compound to toy with. It dissolves readily in solvents like ethylene carbonate and dimethyl carbonate, and this solubility underpins its role as an electrolyte. With a melting point of around 200°C and a molecular weight near 151.9 g/mol, it fits well into existing lithium battery architectures. Its ionic conductivity in solution sits at a sweet spot for lithium migration between the electrodes, allowing batteries to deliver energy without running hot or wearing out too soon.
Technical Specifications & Labeling
Strict labels spell out exactly what buyers get. Battery-grade LiPF₆ usually arrives with purity higher than 99.9%, free of water and metallic impurities that would degrade battery performance. Safety and regulation involve not just purity levels, but packaging that shields against air and moisture. Labels must detail batch numbers, net weight, storage guidelines, and expiration dates. Many countries require UN numbers for transport and hazard codes (like GHS), all because LiPF₆ doesn’t forgive sloppy handling.
Preparation Method
Industrial-scale LiPF₆ comes together through a reaction involving lithium fluoride and phosphorus pentachloride in a carefully controlled environment. Dry HF gas serves as both a reactant and an acid moderator, though its extreme toxicity means the whole set-up needs custom reactors, careful gas scrubbing, and remote operation features. At this stage, there’s no room for shortcuts. Manufacturers use distillation and multi-stage purification systems to chase out contaminants, with the end goal to hit the stringent specs demanded by battery makers. Material from each lot gets sampled, tested, and locked down until it’s proven safe for use.
Chemical Reactions & Modifications
Left in the open, LiPF₆ breaks down with moisture, creating HF, PF₅, and lithium fluoride. This isn’t just a matter of lab tidiness—it has real consequences for people working with it and for the quality of finished batteries. Some development projects focus on stabilizing the electrolyte through the addition of salts or complexing agents, which can reduce side reactions and improve battery shelf life in real-world conditions. Research groups have explored mixing LiPF₆ with additives that mop up the troublesome byproducts. The ongoing challenge for engineers is to preserve what makes LiPF₆ shine—conductivity and voltage range—while dialing down its instability and corrosiveness.
Synonyms & Product Names
If you pick up a safety data sheet, you might see lithium hexafluorophosphate listed alongside alternate names, such as hexafluorophosphoric acid lithium salt, lithium fluorophosphate, or LiPF6. For patent filings and invoices in different countries, these names often overlap. Still, for regulators and customs, “lithium hexafluorophosphate” stays the go-to term.
Safety & Operational Standards
There’s no skirting around the risks. LiPF₆ is toxic and produces HF when it meets water, including air humidity. Workers rely on sealed glove boxes, protective gear, fume hoods, and continuous leak detection. Standard operating procedures focus on avoiding any contact with skin or eyes, and on keeping the work environment bone-dry. Storage calls for double-sealed containers, often kept in nitrogen-purged cabinets. Emergency protocols address not only spills and leaks but the proper ventilation and neutralization of any HF produced. In my years of consulting with battery labs, I’ve seen entire research projects delayed because one batch of salt failed safety screening due to elevated moisture content.
Application Area
LiPF₆ rarely leaves the battery sector. It gets dissolved in carbonates to create electrolytes for nearly every major lithium-ion cell, from those in mobile phones to the battery packs propelling electric cars. Battery manufacturers seek out stable, consistent LiPF₆ to deliver the cycle life and capacity modern devices depend on. Occasionally, LiPF₆ shows up in research on supercapacitors or specialty glass manufacturing, but those markets remain tiny compared to batteries. The link between demand for smartphones, solar power storage, and electric transportation keeps this one chemical at the center of technological progress.
Research & Development
Researchers keep scouring the options for next-generation electrolytes, trying to find the formula that provides the same high performance with better safety and longer lifespan. Some groups explore solid-state alternatives, ionic liquids, or new salts, but LiPF₆ continues to be the standard everyone measures against. Recent patents tackle ways to blend in stabilizers or novel additives to stretch out battery life, especially in electric vehicles where reliability matters most. The challenge remains: find something better without sacrificing what LiPF₆ delivers so well—ionic mobility and compatibility with existing electrodes.
Toxicity Research
Scientists have studied LiPF₆ and its decomposition products for years, looking closely at the threats posed during both normal use and accidents. HF generation stands out as the most severe hazard since it can cause life-threatening injuries on contact and damages lungs if inhaled. The compound itself has moderate toxicity, but its byproducts under abuse or breakdown conditions create most of the concern. Research efforts aim to improve detection methods for tiny HF leaks and to design safer containment, both for large factories and recycling sites. Regulatory agencies set exposure limits for workers, and manufacturers invest in continuous monitoring systems to keep their lines safe and their people out of harm’s way.
Future Prospects
With battery demand climbing, LiPF₆ production keeps scaling up. At some point, the industry faces a fork in the road: stick with what works, or gamble on new chemistry. For the foreseeable future, LiPF₆ drives most of the world’s rechargeable lithium batteries, with incremental improvements making each generation slightly safer and more robust. Next-generation battery projects look to hybrid salts or composites to resolve major drawbacks, hoping to cross the gap between lab success and factory reality. At the same time, better recycling and air monitoring systems could shrink the safety risks tied to large-scale LiPF₆ handling. I expect to see this compound sticking around in high volumes for at least another decade, given its tight integration with current technology and the steep learning curve for replacements. Innovation, in this case, means building on what’s already been proven—pushing for safer work conditions, tighter technical controls, and smarter chemistry in the next batch of batteries.
Why Lithium Hexafluorophosphate Matters
Lithium hexafluorophosphate doesn’t show up in daily conversations, but it quietly powers some of the most important devices we rely on. Pull apart your smartphone, your electric car, or almost any device with a rechargeable lithium-ion battery, and you’ll find this compound working behind the scenes. It’s responsible for carrying the lithium ions between the electrodes, making your phone last the whole day or your car run on electricity instead of gas.
What Sets It Apart in Batteries
For years, battery makers searched for a salt that dissolves well, handles high voltage, and supports the fast movement of lithium ions. Lithium hexafluorophosphate seems to fit the bill. This compound manages to dissolve easily in organic solvents that make up the core of lithium-ion batteries. As it breaks down, it lets lithium ions zip across from one side to the other with hardly any slowdown. That’s how you get fast charging, decent power even as batteries shrink, and better life cycles from your device.
Based on reports from the Argonne National Laboratory and industry leaders like Panasonic, batteries with this salt deliver higher energy density and more reliable performance. Without it, smartphones and electric vehicles would risk short-circuiting or failing after a few recharges. Lithum hexafluorophosphate helps form a thin, protective layer inside the battery, adding stability and preventing nasty reactions or fires that plagued older systems.
Major Uses and Markets
The global demand for lithium-ion batteries has pushed lithium hexafluorophosphate into the spotlight. Since 2020, battery manufacturers in Asia, North America, and the European Union scaled up their orders as electric vehicles took over the headlines. According to market analysis by BloombergNEF, the need for battery-grade lithium hexafluorophosphate grew by over 25% every year since 2018. The compound shows up in everything from wireless headphones and laptops to grid storage packs that help solar power work at night.
As someone who’s tried repairing old batteries, I’ve seen firsthand how much better modern lithium-ion chemistry holds up. Older designs without this salt often swelled, lost charge quickly, and sometimes leaked dangerous chemicals. Modern batteries using lithium hexafluorophosphate last longer, work in more extreme conditions, and rarely fail in catastrophic ways.
The Environmental and Supply Debate
With such widespread use, the industry faces a few tough questions. Large-scale production involves hazardous chemicals like hydrofluoric acid. Mishandling leaks cause environmental damage and pose risks to workers. Health and safety experts in South Korea and China, the world’s top suppliers, have called for more robust controls and transparent reporting.
Battery recycling offers hope, but the technical process for recovering lithium hexafluorophosphate remains tricky. Recent pilot programs in Europe look promising; they focus on capturing the salt during recycling, breaking it down safely, and possibly reusing it for new batteries. As governments push for greener energy, companies must take responsibility for both making and reusing these chemicals without cutting corners.
Innovation and the Road Ahead
The battery market keeps growing, so chemists keep hunting for alternatives. Solid-state batteries hope to sidestep the safety risks while delivering better performance, but they’re not ready for mass production yet. For now, lithium hexafluorophosphate forms a crucial link in our energy chain. Manufacturers, regulators, and users must stay alert to its risks and benefits as the world shifts toward electric everything.
A Closer Look at the Risks
Lithium hexafluorophosphate pops up often in conversations about rechargeable batteries, especially lithium-ion. At first glance, it just seems like another technical ingredient, lost among a dozen chemical names. But the stuff isn’t as harmless as it sounds. This salt, while powering devices from phones to electric cars, comes packed with hazards that people in labs and factories can’t ignore.
Handling lithium hexafluorophosphate brings health risks that most folks don’t see. The compound reacts quickly with water—even the moisture in the air—to produce hydrofluoric acid and other fluorinated gases. Hydrofluoric acid doesn’t just give a nasty burn; exposure, even in small amounts, can turn into a medical emergency. Blisters and burns show up late, which lulls people into a false sense of security. In my college days, our lab supervisor made a rule: goggles stay on and gloves go thick during any work involving this stuff.
Workplace Exposure Isn’t Just a Footnote
People working in battery plants end up on the front lines. Accidental contact or a leaky container means running the risk of skin irritation and respiratory damage. The salts and vapors irritate eyes, lungs, and airways. Over time, even trace exposure has the potential to erode health. Long shifts in battery plant environments can lead to headaches, nausea, and even chronic issues that creep up slowly.
Clean-up after a spill doesn’t just need a mop and bucket. Workers have to gear up with splash-proof aprons, face shields, and proper ventilation. From experience, I’ve seen teams halt production for hours, waiting for specialists with scrubbers and neutralizers to finish the job. Heavy-duty gloves and well-sealed containers aren’t simply “recommended”; they’re mandatory if you want to avoid a trip to the emergency room.
Environmental Risks and Oversight
The environmental side tells a somber story too. If leftover lithium hexafluorophosphate ends up in water or soil, it breaks down into toxic byproducts. Fish and plants suffer. There’s a growing concern about waste from batteries piling up in landfills, leaching chemicals that municipal treatment systems can’t catch. In my city, neighbors worry about what happens around recycling facilities. No one wants to risk contamination in local rivers or parks visited by families every weekend.
Solutions Rely on Smarter Choices
For all the talk of hazards, safer pathways are possible. Better training plays a role: teams that know what danger looks like act faster and more confidently. My own training involved story-driven lessons from old hands who’d seen near-misses and mistakes. Stronger rules and regular checks stop problems before they spread. Improving packaging for shipping and storage brings peace of mind.
Researchers are chipping away at alternatives. Some groups look into salts with less severe side effects. Others focus on containment strategies, using double-walled canisters or absorbent liners. On the consumer side, battery recycling programs need more resources and public support. Dropping spent batteries at certified centers cuts the risk for everyone, from workers to kids playing downwind.
When it comes to chemicals tucked inside devices we use every day, informed choices build safer outcomes. The more communities and companies respect the hidden dangers of lithium hexafluorophosphate, the fewer headlines we’ll see about accidents or contamination scares.
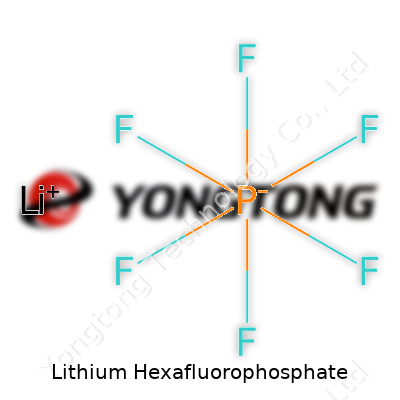
Formula and Structure
Lithium hexafluorophosphate comes with the chemical formula LiPF6. In simple terms, each unit contains a lithium ion (Li+) and a hexafluorophosphate anion (PF6-). In the anion, a phosphorous atom sits at the center, surrounded by six fluorine atoms arranged in an octahedral geometry. These fluorine atoms tightly hug the phosphorous, creating a rigid, compact structure. The resulting compound looks clear and crystalline, often appearing as a white powder.
Why This Compound Matters
Anyone who has cracked open a smartphone or an electric vehicle battery might feel puzzled about the quiet heroics of lithium hexafluorophosphate. This salt isn’t just a chemical oddity—it plays a crucial role in the performance and safety of lithium-ion batteries. From my time double-checking datasheets on popular battery chemistries, I learned that most commercial lithium-ion cells rely on LiPF6 as the go-to electrolyte salt.
It isn’t just simple preference. LiPF6 dissolves easily in blends of organic solvents like ethylene carbonate and dimethyl carbonate, letting lithium ions move freely and reduce resistance inside the battery. The salt’s chemical balance not only enables stable voltage but combats unwanted reactions that could trigger leaks and fires. A paper published in the Journal of Power Sources highlights that LiPF6 has become standard partly because of its ability to generate a stable, thin interface (solid-electrolyte interphase) on the battery’s anode. Without that, a battery drops voltage or ages in weeks.
Risks and Looking for Solutions
Still, real-world chemistry finds tradeoffs. Ask anyone who has dealt with battery leaks or thermal runaway, and they will tell you about the explosive side of LiPF6. The compound reacts with trace water to produce toxic hydrofluoric acid (HF) and other nasty byproducts. Even small mistakes in humidity control during cell assembly make batteries corrode or fail before leaving the factory.
As global demand for rechargeable devices grows, manufacturers and researchers chase safer, more robust alternatives. Some teams test salts like lithium bis(fluorosulfonyl)imide (LiFSI), which won’t break down as quickly when wet. Others develop advanced coatings on electrodes to fend off the breakdown products of LiPF6. These efforts draw from chemical knowledge pieced together over decades, such as the role of fluorine-rich environments in managing battery stability.
Looking through research from places like Argonne National Laboratory underscores this push. Their teams keep refining the recipe for battery electrolytes—either by tweaking the fluorine content, stabilizing the interface even further, or designing entirely new compounds. While cost and large-scale manufacturing challenges remain, the blueprint comes from understanding what makes LiPF6 tick…and what makes it fragile.
Reaching a Practical Balance
People keep expecting longer-lasting, safer, and cheaper batteries. That pressure keeps LiPF6 in the crosshairs, both for its reliability and its chemical quirks. The right formula isn’t just a stroke of lab luck, but the outcome of balancing real-world risks and benefits. As more teams put their heads together, chemistry keeps pushing the threshold for what’s possible in energy storage.
Why Safe Practice Matters
Every battery revolution depends on careful chemistry. Lithium hexafluorophosphate has become standard for lithium-ion batteries, but this salt brings more than charge—it brings risk. Moisture changes it to hydrofluoric acid, one of the nastiest substances I’ve ever learned about. Hydrofluoric acid eats through skin, sinks deep, and can be fatal. For me, a healthy respect for material safety grew out of sweaty afternoons in old school labs, watching how a single drop on unprotected hands halts class. I never forgot that chill. The lesson stays with me when handling any volatile compound, especially ones feeding a tech boom.
Storage Requires More than Labels
Lithium hexafluorophosphate doesn’t forgive sloppy storage. Dry, air-tight containers win the day; humidity slips in, and that dangerous acid forms. My colleagues often argue about spending a few extra dollars on certified desiccators or specialized cabinets. I’ve seen too many crisp white labels on cracked jars in dusty storerooms—cutting corners never makes sense once you understand the risks. Actual safety demands more than just locking things up. It takes regular checks, clean seals, and silica gel refreshed before it sits useless at the bottom of the container.
I like simple systems: storing this salt only below 30 degrees Celsius, away from any hint of moisture or incompatible chemicals like strong acids or oxides. Fire-resistant cabinets, kept in a cool, ventilated room, form a smart barrier. Ventilation stops fume buildup if leaks happen. All that’s needed for bad events is one careless stack near an HVAC return or a forgotten beaker of water on the same shelf. I’m not a fan of “set and forget”—auditing inventory, checking expiry dates, and recording all movement makes a big difference. Digital logs help track everything without losing paperwork in the shuffle.
Personal Protection Isn’t Optional
Handling this salt, I picture my old chemistry teacher with his stern face over safety glasses. Nitrile gloves, thick goggles, and a lab coat are non-negotiable. Splashing acid burns in seconds. Vapors can irritate eyes and lungs before you even notice. Having a good fume hood is my practical answer for prepping solutions or measuring out powder. There’s no pride in skipping steps—comfortable goggles or disposable sleeves set the standard, especially for anyone handling lithium hexafluorophosphate in bulk. Once, a rush to finish a late-night test left a friend with a nasty skin rash, a reminder nobody expects until their own time comes.
Accidents and Emergency Response
I’ve been close to serious near-misses. Quick access to calcium gluconate gel for skin exposure and eyewash stations saves more than time—it prevents scarring and long-term damage. Emergency instructions need to stay within arm’s reach, not buried behind old binders. Training drills boost muscle memory and keep people honest. I trust written protocols paired with strong safety culture, reinforced through real stories of accidents. No one stays casual once they know what hydrofluoric acid can do. Pairing experience with up-to-date safety sheets and practical tools empowers teams to catch mistakes before they cascade.
Continuous Learning and Responsibility
Every year, new staff need refreshers. I’ve noticed people let safety slack when working with something every day. Supervisors and teams must share responsibility. Keeping lithium hexafluorophosphate safe means more than compliance; it means personal involvement, shared reminders, and respect for risks most folks never see. We owe it to ourselves, our colleagues, and the wider community. Honest communication and a willingness to improve keep accidents rare. That’s the bottom line in handling dangerous materials—either as a researcher, a manufacturer, or anyone supporting the energy systems that power our lives.
The Crucial Role of Purity in Battery Chemicals
Quality matters a lot in battery chemistry. Lithium hexafluorophosphate, or LiPF6, finds its main use as a salt in the electrolyte solution that flows between the electrodes in lithium-ion batteries. Devices from smartphones to electric cars rely on the stable performance of their batteries. Even for those who don’t build batteries for a living, it’s clear that one faulty part can sideline a whole device or vehicle. Just like bad gasoline can ruin an engine, impurities in a battery electrolyte can cause a world of headaches—think short circuits, gas buildup, or even fires.
Purity Grades: What Manufacturers Offer
Lithium hexafluorophosphate comes in several grades. Each grade matches a specific end use. For the battery market, the highest purity counts most. On the shelves, you’ll usually find battery-grade and industrial-grade. Battery-grade, which carries the most demanding standards, holds purity at 99.99% or higher. Even small amounts of water, metals, or particle contamination can mess with charging cycles and lower battery lifespan. I’ve seen engineers test samples and toss out any lot that shows a spec of unexpected residue.
The lower tier—industrial-grade—sits around 99% purity or above. It’s fine for specialty chemicals or research, but not a safe bet for making high-performance batteries. Some research labs will use it if they’re chasing cost savings or studying new battery designs, but they always flag their results since side reactions sneak in more easily.
Specifications That Matter
Purity alone doesn’t tell the full story. Chemical and physical specifications spell out what’s really inside the bag.
Water content proves especially important. LiPF6 reacts with water to form toxic hydrogen fluoride and lithium fluoride—both of which can degrade battery components quickly. Stringent specifications keep water content to less than 50 parts per million (ppm) in battery-grade material. If humidity creeps in, safety testers will catch it.
Metal ion contamination stands out as another serious concern. Iron, sodium, calcium, and magnesium—these trace metals can come from raw materials or even drum linings. Companies typically require total metal content lower than 10 ppm. Any spike in these elements raises red flags, since metals like iron can speed up unwanted side reactions in electrolytes.
Acidity and residue round out the quality checklist. Bad batches sometimes include lingering acid residues or detectable solids after dissolving the crystals. A simple pH check, residue-on-ignition test, and color clarity scan will weed out poor-quality product.
Why These Details Count for Battery Performance
Every time a battery charges or discharges, chemical reactions run back and forth through the electrolyte. If extra ions or water sneak in, the chemistry takes a wrong turn. Heat builds, cells gas up, and sometimes performance tanks years before it should. High-grade LiPF6 helps manufacturers reduce risks and offer warranties people trust.
Raising the Bar on Purity: The Path Forward
As electric vehicles hit the roads in record numbers, the spotlight on lithium battery safety and lifespan only grows. Better process controls and improved analytical methods, like inductively coupled plasma mass spectrometry, give labs more precision. Some companies have also moved storage and handling under dry rooms with strict monitoring, cutting down on water exposure even before a drum leaves the warehouse. Anyone serious about safe, long-lasting batteries knows that purity and careful specification checks are non-negotiable—not just nice-to-have.