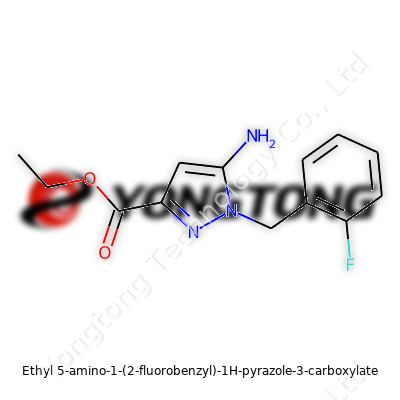
Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate: A Grounded Look at an Emerging Compound
Historical Development
A few decades ago, synthetic chemists started playing with pyrazole rings, not just for curiosity, but because once in a while, you run into a structure that does something remarkable. Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate came out of this tradition, shaped by the hunt for molecules capable of doing heavy lifting in pharmaceutical settings. Labs first synthesized it as part of research into fluorinated benzyl derivatives, hoping the pyrazole core would bring activity against biological targets, maybe serve as a lead scaffold for new drugs. As I’ve seen while working on similar scaffolds, the drive wasn’t just about creating something novel — teams chased better selectivity, metabolic stability, and biological engagement, a mindset that pushed development from gram-scale routes in university labs to high-throughput screening in biotech partnerships.
Product Overview
Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate brings together several functional groups that catch the eye of chemists and pharmaceutical researchers. You’ve got an ethyl ester, which can often serve as a handle for further chemical tweaks or prodrug strategies. The 5-amino substitution plays into hydrogen bonding games inside enzymes or receptors, while the 2-fluorobenzyl group isn’t just window dressing — fluorine’s electronegativity changes how the molecule fits in protein pockets, sometimes making the difference between potency and inactivity. In my own work, seeing a fluorinated aromatic inserted at this position usually signals that folks were thinking about metabolic stability and binding interactions from the start.
Physical & Chemical Properties
The molecule presents as an off-white, even slightly yellowish, crystalline powder. Under the microscope, crystals form needles or small plates, and unless humidity goes off the rails, they keep pretty stable at room temperature. Typical melting point dances around 120-129°C, depending on batch purity, and the product goes from soluble in organic solvents like DMSO or DMF to moderately soluble in ethanol, but won’t budge much in cold water. The fluorobenzyl ring pumps up the lipophilicity, so if you’re running extractions, hexane or ethyl acetate do a decent job. Chemical reactivity stems off the pyrazole, but the 5-amino site loves to act as a nucleophile, and the ethyl ester holds up through mild acidity but suffers under basic or strongly acidic hydrolysis. In analytical labs, the compound gives a sharp NMR fingerprint; proton and carbon signals reflect the electron-withdrawing punch of fluorine and the signature coupling constants that pyrazoles usually show.
Technical Specifications & Labeling
Lab catalogs typically list this compound at purities above 98%. Labels have to spell out all functional groups because that tells you what sort of handling is required. MSDS data tie-in tightly: keep the powder dry, avoid skin contact, and treat spills with the respect you’d give similar aromatic amino pyrazoles. Batch production involves repeatedly running HPLC and NMR to confirm identity, especially since impurities—like unreacted benzyl or hydrolyzed esters—can mess with bioassay results. Storage calls for sealed amber glass, away from light and moisture, kept at 2-8°C—cold but not frozen. Container labels highlight hazard codes, signal words for lab techs who might be in a rush, and always a clear note about the aminopyrazole’s irritancy risk.
Preparation Method
Stepwise synthesis usually kicks off with 2-fluorobenzyl bromide and a pyrazole precursor. I’d set up a substitution in anhydrous DMF with a base like potassium carbonate, making the N-alkylated intermediate. The ethyl carboxylate groove comes from either condensation or alkylation, and the introduction of amino groups is most often achieved through selective reduction of nitro precursors or direct nucleophilic substitution. Each stage needs careful workups, typically a water-organic extraction, brine wash, and a lot of column chromatography before crystallization. The final compound gets checked on TLC, followed by spectral verification — never skip running an LC-MS, because even small byproducts can complicate downstream studies or patent claims.
Chemical Reactions & Modifications
The aminopyrazole core stands up to a variety of transformations, and in my experience, teams rarely leave it unmodified for long. You can acylate the 5-amino group to make amides, introduce sulfonyl groups, or couple with activated esters for building new analogs. The ethyl ester function turns into a carboxylic acid by hydrolysis, which then opens doors for peptide couplings or attaching fluorescent tags. The fluorobenzyl sidechain remains pretty stubborn (that’s fluorine for you), but aromatic substitutions on the ring or hydrogenation steps can alter the molecule’s pharmacokinetic profile if the project calls for it. Analytical chemists stay busy tracking these modifications since even a slight change at the amino group can flip the way a molecule behaves in biological systems or in an LC-MS assay.
Synonyms & Product Names
Chemists love shorthand almost as much as acronyms. In practice, I’ve seen this compound go by “2-Fluorobenzyl aminopyrazole carboxylate,” “5-Amino-1-(2-fluorobenzyl)pyrazole-3-ethylcarboxylate,” and regional suppliers sometimes list it with lab-specific codes depending on the research pipeline. These synonyms are not just about making cataloging easier—they help during literature searches, especially tracking bioactivity data and structure-activity relationships published under different project names or countries. Catalog entries, patents, and publications all list variations, so a thorough researcher needs to cross-check any learning effort about pharmacology or toxicology.
Safety & Operational Standards
Despite lacking the infamy of some legacy chemicals, this compound draws the same caution during handling as any aminopyrazole derivative. Even a small whiff while weighing out grams in a fume hood reminded me how volatile some intermediates can be. Vapor and dust could irritate eyes and airways, so goggles and nitrile gloves make sense on every batch day. Waste solvents, especially DMF and DMSO laced with the molecule, require collection as hazardous byproduct rather than pouring down public drains. I always encourage teams to run practice fire drills in any lab dealing with aromatic esters – it rarely comes up, but an accidental spill over a hotplate can catch technicians off guard. Modern standards call for spill kits, eye washes, and clear SOPs outlining what to do if contact occurs.
Application Area
Most real interest in this molecule arises from its promise as a pharmaceutical intermediate and experimental agent. The pyrazole backbone invites exploration for anti-inflammatory, antimicrobial, or anticancer effects, while the position of the fluorine atom matters for metabolic routes inside animal models. I’ve seen papers testing similar derivatives against both kinase targets and CNS receptors. Some researchers use this scaffold as a launchpad for SAR (structure-activity relationship) campaigns, particularly when designing inhibitors that need both potency and metabolic resilience. Pilot studies also explore agrochemical routes, searching for compounds safe enough for crops but rough on insect pests. In industrial settings, process chemists push to scale up the synthesis only if early biological screens justify the investment.
Research & Development
My conversations with medicinal chemistry teams turn again and again to how quickly analogs can be made and tested. This compound stays popular because of its chemical tractability — you can swap out groups or attach probes using tried-and-true lab tricks, then run them through a suite of target enzyme assays. I’ve followed academic groups publishing tweaks that add bulk at the benzyl position, or introduce electron donors to the pyrazole, all hunting for better receptor binding or longer half-lives in mouse models. Collaboration between universities and industry speeds up iteration, and every new analog gets sent through panels of in vitro and in vivo assays to spot toxicity, bioavailability, and potential off-target action.
Toxicity Research
Toxicology studies cross into animal and cell-based systems, probing acute and chronic response to the compound. Researchers measure LD50 in rodent models, run genotoxicity screens, and check for any mutagenic effects using established bacterial assays. Early data point toward moderate acute toxicity—less than some older pyrazole derivatives, but not enough to ignore regulations. Metabolic studies track the fate of the fluorobenzyl group, since aromatic fluorides sometimes form reactive intermediates in human livers. Dermatology teams test for sensitization using classic guinea pig or patch tests. With each study, safety data expands, forming the backbone for regulatory approval if novel drug candidates reach clinical stages.
Future Prospects
Looking ahead, this compound’s chemical flexibility and diverse functional groups set it up for continued exploration. I expect to see more analogs targeting inflammation, CNS disorders, or microbial pathogens, especially as machine learning begins predicting subtle modifications for improved pharmacokinetics or reduced side effects. Intellectual property filings already point toward new uses outside pure pharma—plant science, material coatings, and even as specialty ligands for imaging purposes. Through all these developments, researchers keep circling back to the balance between synthetic accessibility, safety in the lab, and effectiveness in biological settings. I see promise in modular synthesis—methods capable of producing a library of analogs without needing a new route each time. This approach speeds up discovery and, more than anything, provides the breathing room to chase drug candidates with a better chance of helping patients or solving agricultural needs.
Getting Under the Hood of a Modern Molecule
Chemistry textbooks overflow with structures that look like squiggly lines and foreign characters to most people. When you break it down, each part of a name tells a story about function, origin, and how researchers interact with the world. Owning some background in organic chemistry, I notice that substances like Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate do more than just fit on a shelf—they shape new treatments and technologies.
Translating the Name Into a Structure
This compound’s core is a pyrazole ring, made up of three carbon atoms fused with two nitrogens, stitched together in a five-membered loop. Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate, as the name suggests, is a maze of specific attachments:
- An ethyl ester at position 3 connects to a carboxyl group, offering a handle for reactivity and solubility.
- Position 5 houses an amino group—think of this as a point that can interact through hydrogen bonding, or become a target for further chemical tweaking.
- At position 1, a 2-fluorobenzyl group latches onto the pyrazole, delivering a bulky but precise piece. Fluorine changes electron distribution, often used to boost metabolic stability in drug design.
Piecing all this together, the structure winds up as a dance of rings, amines, and esters, each chosen for a reason. Every substitution, every atom, answers a question: How does this piece improve function? What will it resist? Where might it break down?
Importance in Today’s Chemical Toolbox
Why care about a single pyrazole derivative? Beyond lab benches, these types of molecules stand at the frontline of pharmaceutical and agrochemical discovery. Subtle changes—like adding a fluorine atom—can mean the difference between a medicine that works and one that misses. My own hands-on work in a synthetic organic lab showed me how small tweaks, like swapping an amine for another group, can transform yield, health effects, or shelf life.
This particular structure, with its aromatic benzyl tail and reactive amine, suggests activity in biological systems. Researchers chase molecules like this for anti-inflammatory, anticancer, or antifungal candidates. The ester gives drug designers a way to make prodrugs—versions of medicines that activate once inside the body, reducing side effects on the way in.
Balancing Promise and Caution
Chemical design holds promise, but every step raises questions about safety, sustainability, and cost. The use of a fluorine atom often extends life in the body but can also mean sludge in the environment. Downstream effects from tiny changes turn up all the time; publications catalog surprises from unexpected metabolic byproducts or toxicity. Addressing these risks requires commitment to testing, open data, and sharing across disciplines. Chemists need tools that predict outcomes more precisely so fewer surprises land in late-stage trials or, worse, in patient outcomes.
Labs would do well to partner with environmental chemists as early as possible, long before a candidate leaves the flask or pilot plant. More green chemistry methods, like less toxic reagents or recyclable catalysts, help lighten the footprint. Bringing in early-stage toxicity screening saves resources by flagging problems before scale-up.
Final Thoughts
This molecule’s structure holds more than technical curiosity—it marks a challenge and a responsibility for every hands-on chemist or designer. Choices made at the drawing board ripple forward, leaving marks in science, medicine, and the world beyond the page.
The Workhorse for Drug Discovery
I’ve spent quite a bit of time around small molecule labs, and whenever chemists discuss new drug scaffolds, certain building blocks start showing up again and again. Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate (let’s just say “this pyrazole” for now) frequently surfaces in conversations about pharmaceutical candidates. It’s no mystery. The core structure lends itself to useful tweaking—those who’ve tried swapping out side-groups know how much of a win it can be if a single change sharply tweaks biological activity.
Drug development moves slowly and demands serious imagination. Each new molecule, especially those with a pyrazole ring like this, opens doors to targets in diseases that stumped yesterday’s treatments. The fluorobenzyl group can help molecules cut through biological barriers and reach tough areas in the body. Medicinal chemists often use this piece to explore anti-inflammatory, antiviral, or anticancer leads, tweaking it along the way. Publications and patents tell a story of researchers leaning on this scaffold for scaffolding new kinases or CNS modulators. Some chemists rely on this building block for SAR studies—comparing small changes and seeing which ones hit the mark for biological effect.
Tools for Chemical Biology
One reason this pyrazole remains so popular involves its role as a “jumping off” point for advanced intermediates. You’ll see folks in academic labs churning out dozens of analogs during hit-to-lead optimization. The amino function gives flexibility for making a new generation of probes aimed at mapping how human proteins interact with small molecules. Over the years, I’ve watched PhD candidates paint pathways using derivatives just like this. These experiments bring fresh understanding for which targets respond to molecular tweaks, mapping the science for future therapies.
Chemistry Beyond Pharma
I’ve seen this molecule outside strictly medical labs as well. Agrochemical research—pesticide and herbicide development in particular—sometimes leans on fluorinated pyrazoles. Certain molecular tweaks can help disrupt pests’ enzyme pathways more efficiently. Formulators test a string of compounds like this to balance effectiveness and environmental safety. The potential for pesticide resistance grows year after year, so new scaffolds aren’t just valuable—they’re essential for resilient crops and yields.
Barriers and Responsible Use
Synthesizing advanced compounds isn’t a risk-free business. Safety and environmental impact can’t be left as an afterthought. As more labs order in custom fluorinated pyrazoles, the environmental footprint of their manufacturing has become a talking point in green chemistry circles. Some companies lean into cleaner reactions with less waste and hazardous byproducts. I encourage academic labs and contract manufacturers to pick greener methods whenever practical; atom-economical transformations benefit everyone downstream, whether they work in research or live near factories. Scaling up always exposes new risks—R&D leaders have an obligation to trace any byproducts or persistent chemicals in discharge water.
Eyes on the Future
It’s not just about what a compound does today. Fact-based oversight, transparent sharing of safety studies, and sharing synthetic procedures make the difference between solo wins and progress for all. Supporting new scientists in mastering these chemistries not only trains the next generation of problem-solvers, it multiplies the pool of ideas. Encouraging collaboration across disciplines—medicinal, environmental, and industrial—usually unlocks uses none of us expected.
What Purity Really Means for Your Work
Lab projects can stumble over impurities, so it makes sense to check the chemist’s favorite metric: purity. Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate usually appears at a purity of 97% or greater when sourced from reputable suppliers. Some vendors push for 98% or even 99% options, but the standard hovers around 97%. I’ve seen colleagues scramble to troubleshoot wonky results, only to find the culprit was a slight dip in compound purity—evidence that taking shortcuts here rarely pays off. Purity directly affects results across pharmacology, materials science, and early-stage drug discovery, often determining whether an experiment moves forward or sends you back to square one.
Buying a lesser-known intermediate like this compound means you want assurance on quality. Good vendors send detailed certificates of analysis (COA), not just numbers on a web form. An authentic COA gives you data about key impurities, water content, melting point, and batch details. Demand transparency. My own experience with lower-grade materials taught me that guesswork doesn’t save money in the long run. Unexpected contaminants can show up as false signals in analytical methods like NMR or LC-MS, knocking even well-designed projects off-beam.
Packaging Sizes for Real Workflows
Lab needs vary. Someone investigating a new synthesis route might want a tiny sample, while others developing a drug candidate could need a kilo or more. The standard packaging sizes for ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate reflect that reality. Most suppliers offer packs beginning at 100 milligrams or 250 milligrams. For early-stage research, a gram-style bottle—between 1 and 5 grams—hits the sweet spot. Once a project starts scaling, 10-gram and 25-gram options pop up. Commercial buyers sometimes ask for custom bulk quantities, 100 grams up to a kilo, with packaging adjusted for safe transit and minimal material loss.
Getting packaging right matters. In my own research, large containers for small-scale work often led to product loss or contamination, a costly lesson. Most reputable suppliers seal these compounds in amber vials or HDPE bottles, often under nitrogen or with desiccants, to protect against light, moisture, and slow hydrolysis. Mishandling even small amounts can spoil the batch, so having available sizes reduces waste and saves dollars down the road. The better suppliers also listen to requests for custom pack sizes or special storage.
Who Sets the Standard?
International standards help buyers judge a supplier’s credibility. Vendors with ISO certifications or those who publish third-party lab results tend to attract clients who insist on traceability. I’ve worked in labs where traceable sourcing stamped out uncertainty, saving hours of backtracking. Regulatory compliance feels bureaucratic, but it becomes a lifesaver for teams dealing with high-value intermediates, especially when clinical or patent filings rely on reproducible results. You end up with fewer headaches and greater confidence in published data.
Practical Fixes for Sourcing Issues
Order only the amount you reasonably expect to use within a project’s timeline. Don’t just chase a bargain on a big bottle. Ask suppliers for full certification, storage advice, and samples if you’re scaling up for the first time. Track batch numbers and test aliquots on arrival, rather than discovering problems halfway into your workflow. Reach for reputable suppliers, and don’t shy away from asking direct questions about chain of custody and pricing transparency. Reliable data starts with reliable materials, and good sourcing practices ripple through every corner of your research or manufacturing chain.
Why Storage Matters with Sensitive Chemicals
Handling chemicals involves more than working inside a lab and jotting down notes. I remember the early days of my research career, shuffling through an old storage cabinet in search of a bottle, only to realize that careless storage had ruined a new batch. Over time, I’ve seen how correct storage keeps compounds stable, helps research run smoothly, and above all, keeps everyone safe. With something like Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate, a controlled approach to storing it makes a real difference between good science and disaster.
Temperature Sets the Stage
Most organic compounds, especially those with delicate functional groups like amines and esters, prefer a cool home. This pyrazole derivative holds up best when kept cold, ideally between 2°C and 8°C, which lines up with refrigerator conditions in most labs. At room temperature, chemicals can decompose or react with moisture from the air. In some cases, I’ve seen unexpected color changes, indicating the start of breakdown earlier than expected. By keeping the bottle consistently cool, you slow down processes that degrade its quality. Labs often rely on dedicated chemical refrigerators with tight temperature ranges to avoid thermal swings, something a kitchen fridge rarely offers.
Shielding from Light
Light triggers reactions in many organic compounds—not always visible right away, but the damage sets in. Pyrazole rings don’t always play nice with UV or strong light, so dark storage matters. Brown glass bottles work well, as does keeping the compound inside opaque secondary containers. Years ago, I learned the hard way while storing samples in clear containers; even a few hours under the bench lamp led to a ruined experiment. Simple steps—covering bottles or using secondary boxes—kept samples usable and cut down on frustration during repeat trials.
Dry Environments Keep Trouble Away
Moisture in the air can break down esters, so humidity control shouldn’t be brushed aside. Even leaks in a storage fridge or that drip from the HVAC vent above can wreck purity. I’ve had colleagues lose days of work due to a forgotten desiccant pack. Keeping the container tightly sealed, and ideally placing it in a desiccator or adding silica gel packs inside the storage box, can help. Many labs use dedicated humidity meters to check the air in storage rooms. If powder clumps up or liquid separates, take it as a warning—something likely crept in.
Labeling and Access Controls
Correct labeling stops mistakes before they start. Every bottle should show not just the name, but the date opened, concentration, and any risk warnings. I’ve watched new staff grab the same bottle meant for another trial, losing both samples to cross-contamination. Arranging chemicals by hazard class and logging every removal and return builds accountability. Locks on refrigerators and written sign-out logs provide real barriers to mishandling or mixups. It only takes one accident to cost months of progress or worse, put someone at risk.
Disposal and Expiry Considerations
Every chemical faces an expiration. Tracking dates and scheduling audits matter. Disposal needs careful planning, following local regulations to prevent environmental harm. Some labs run regular training to remind staff that expired or degraded chemicals can become more dangerous. Never pour these substances down the drain, and never throw them in regular trash—eco-conscious disposal protects both public health and the reputation of everyone involved.
Understanding Chemical Hazards Up Close
Working with organic chemicals like Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate has never felt routine, no matter how comfortable a lab bench might seem after years of use. Tools and habits mean little until faced with new substances—this one is a mouthful and not a household name for good reason. Its structure points to traits I recognize from related compounds: potential toxicity, possible irritation, and tricky storage conditions. Even if a chemical doesn’t grab headlines, each one can introduce big risks.
Skin, Eyes, and Inhalation—The Usual Suspects
Direct contact leaves room for harmful reactions. Most labs, including mine, treat new or uncommon pyrazole derivatives as potential irritants. This compound checks that box. Splashing solutions or powders onto skin—sometimes it takes just a drop—leads to itching, redness, or stinging. Eyes offer even less protection, and nobody enjoys an eyewash fountain. Standard PPE isn’t optional here—nitrile gloves reach up the sleeve, goggles hug tight to the face, and a lab coat often stays buttoned through the day.
Airborne risk comes from both powders and vapors, especially during weighing or heating. Fume hoods handle most of the load. Having placed too much trust in a “quick” operation before, I learned how vapors creep up. Respirators make sense when ventilation isn’t guaranteed, but my first choice is still a working hood.
Fire and Reactivity Risks
A lot of organic esters and pyrazoles ignite if a spark catches them off guard. Ethanol and fluorinated benzyl groups often bring flammability to the table. Open flames, hot plates, static discharge—any of these can set off a chain reaction if a flask cracks or solution boils over. Small-scale synthesis rarely means small-scale consequences. I’ve always waited to label flasks until after chemicals are safely inside, but keeping water, sand, or a fire extinguisher handy calms the nerves whenever dealing with volatile organics.
Storage matters too. Avoiding excess heat and keeping incompatible chemicals apart sits among the most basic but forgotten moves. A student once left a mixed waste container capped without a vent, and it bulged overnight. Ventilated cabinets prevent those surprises, particularly for materials like this one.
Spills and Disposal—No Shortcuts
Lab spills tend to inspire panic, but a plan turns that panic into action. Absorbent pads, baking soda, and dedicated waste bins belong within arm’s reach. From experience, a delayed cleanup always complicates everything—think longer exposure and harder stains. Disposal needs a dedicated route. Diluting or washing this kind of chemical into the sink crosses both legal and ethical lines. Hazardous waste collection, with clear labeling, protects custodians and downstream handlers.
Documenting and Communicating Risks
Chemical safety lives in the details. Keeping MSDS sheets handy protects newcomers and seasoned chemists. Training sessions serve as the foundation, but so do routine checks and honest discussions about near-misses—blame never solved anything after an accident. Updates come from respected sources like PubChem and journal articles rather than hearsay, and reviewing them before ordering or synthesizing new compounds has spared my teams headaches time and again. Strong habits don’t happen by accident; they build from stories and shared responsibility.
Building a Safer Lab Culture
Physical precautions play one part, but culture shapes everything. In every lab I’ve joined, senior researchers showed that safety wasn’t paperwork—it was a way of working. Regular practice, respect for the unknown, and open comments on procedures set the tone. Getting it right on the little things prevents big disasters. Chemical handling, especially with molecules as complex as Ethyl 5-amino-1-(2-fluorobenzyl)-1H-pyrazole-3-carboxylate, proves every day that details matter. Even on a quiet bench, nothing feels routine.