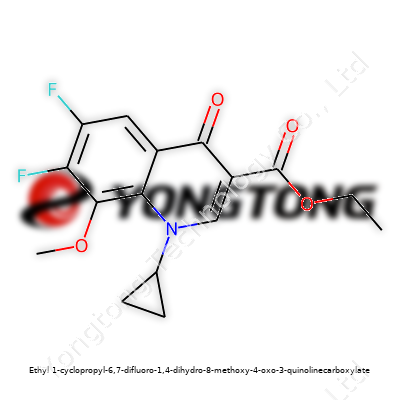
Commentary: Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate – Beyond its Chemical Formula
Historical Development
Long before modern medicine leaned heavily on synthetic chemistry, natural remedies provided basic care. As the 20th century pressed on, broad-spectrum antibiotics pushed out many of those home remedies. The discovery of quinolone derivatives shook up pharmaceutical development. Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, a mouthful for anyone but a chemist, represents decades of incremental improvement on these original scaffolds. Scientists didn't land on this structure with a single stroke. Instead, university labs and pharmaceutical teams tested hundreds of side chains, aiming for compounds that could bypass bacterial defense and improve absorption in the body. The cyclopropyl group at position 1 and dual fluorines along the ring structure didn't show up by accident — they were outcomes of years spent testing subtle tweaks for better activity or fewer side effects. These development cycles usually outpace textbook timelines and involve cross-border collaborations that don't always get much recognition.
Product Overview
Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate stands at a crossroads between chemical curiosity and practical medicine. Experienced chemists see more than a name. They know its scaffold fits the fluoroquinolone family, which signals strong antibacterial potential and a history of impact in infectious disease work. Development in both labs and industry sometimes races ahead of the clinic, but this molecule's path shows the deliberate marriage of synthetic chemistry and pharmacological goals. Its ethyl ester group gives medicinal chemists a handy hook for further transformations, and its dense ring system encourages binding to biological targets. Managing its reactivity and formulating it in ways that make sense for patients reveals the difference between just making molecules and delivering treatments.
Physical & Chemical Properties
Unlike table sugar or salt, which dissolve predictably and offer clear melting points, molecules like this demand real lab work to characterize. Depending on purification and storage, the physical form shifts between amorphous powders or crystalline solids, each with implications for stability and formulation. The difluoro substitutions and methoxy group change polarity, making solubility and absorption less straightforward than a superficial glance at the formula might suggest. Cyclopropyl branching also adds rigidity, changing how the molecule fits with others or resists metabolic breakdown. Density, melting range, and partition coefficient all influence how this substance behaves in research settings — and later, in real-world treatments.
Technical Specifications & Labeling
Through years in various analytical labs, I’ve learned firsthand that repeatability matters most. Users need technical sheets that provide purity profiles, assay data, and contaminant levels — not marketing fluff. Anyone involved in pharmaceutical manufacturing gets a crash course in these practices. Detailed specifications cut down on guesswork. Inclusion of lot numbers, expiry dates, and batch analysis marks the difference between lab-bench experimentation and industry-ready practice. Even the smallest change in impurity levels, moisture content, or physical form can flip safety profiles on their heads. Regulators scrutinize these details, and so should anyone handling the compound. Mishandling or mislabeling often starts with the little things that seem too trivial to check until they trip up an entire research program or clinical trial.
Preparation Method
Most lab syntheses for this type of quinolone ester follow multi-step procedures requiring careful temperature control, solvent selection, and exact reagent ratios. Cyclopropyl introduction often occurs early in the sequence, using cyclopropylamine or its derivatives, followed by difluorination, ring closure, and esterification. Even routine steps like purification challenge chemists, especially scaling up from grams to kilograms. I’ve seen reactions run smoothly in ten-gram batches that caused chaos at larger scales due to changes in heat transfer or solvent evaporation. Sometimes, a tiny tweak in a reaction step (switching solvents, changing addition order) brings yields up from frustration to production. Each researcher handling scales or solvents, from undergraduate students to process engineers, owes their results to reliable procedures worked out across years and generations of lab work.
Chemical Reactions & Modifications
Quinolone esters like this don’t sit still in a flask. They act as versatile platforms for chemical modification. Modifying the ester, swapping out substituents on the ring, or attempting partial reduction all create spinoffs that serve as leads for new pharmaceuticals or agricultural products. Adding electron-rich groups or stripping away halogens produces families of related molecules, some deadly to bacteria, some screens for entirely new uses. Techniques range from basic hydrolysis all the way to advanced palladium-catalyzed couplings. With every experiment, chemists learn more about which changes tip the balance toward potency, solubility, or reduced toxicity. One forgotten lesson: rushing into modification work before proper purification often leads to confusing results, wasted resources, and years lost retracing old steps.
Synonyms & Product Names
In any global field, especially pharmaceuticals, names and synonyms pile up with each company, region, or academic publication. Some refer to this compound by code names, others by systematic IUPAC terminology, and still others by trade monikers connected to broader projects. These aliases highlight the challenge of cross-checking research results or sharing findings beyond local circles. Mislabeling due to synonym confusion led to more than one batch of wasted reagents in my own lab days. Databases and international standards try to keep pace, but practitioners still rely on experience and double-checking, especially when moving between suppliers.
Safety & Operational Standards
Chemical hygiene matters more than ever. Whether handled by academic researchers, scale-up professionals, or students, this compound brings hazard concerns that shouldn’t be glossed over. Fluorinated organics, by their very nature, demand special disposal methods to prevent long-term environmental impact. Direct contact can cause irritation, inhalation risks grow during manufacture or scale-up, and accidental spills create chain reactions beyond the first responder. Years in chemical plants taught me never to skip safety training, even for experienced teams. Clear labeling, airtight standard operating procedures, and up-to-date safety data sheets keep accidents rare but also ensure quick responses when things go wrong. Investing time here pays off not just in regulatory compliance but in lives and livelihoods preserved.
Application Area
Most people outside the chemical sciences miss the subtle impact molecules like this have on disease treatment, agriculture, and even material science. In hospitals, it signals new potential for tackling persistent bacterial infections, especially as resistance outpaces more conventional options. Labs designing antimicrobial coatings for medical devices keep an eye out for derivatives that resist biofilm growth. In the hands of agricultural researchers, minor chemical rearrangements translate to new defenses against crop pathogens. Each field, from human health to animal care or plant protection, often circles back to the original scaffold, looking for that balance of cost, impact, and side effect profile. Commercialization only happens when all these pieces lock together.
Research & Development
Every year sees a flood of research aimed at outsmarting evolving bacteria. Projects focus as much on ways to modify this compound as on ways to deploy it. My conversations with academic researchers reveal two big trends: using this scaffold in combination therapies, and applying computational tools to speed up modifications that could fight resistance. Industry brings money and tools to the table, but basic research still lays the foundation. Open-access journals grow thicker by the month with new analogs, patent applications, or clever tweaks that might never make it to market, but each adds to the collective knowledge bank. At the same time, collaboration across national borders increases, tied together by digital platforms that didn’t exist a generation ago.
Toxicity Research
Stories from hospital pharmacists and toxicologists routinely remind me that even wonder compounds carry risks. Fluoroquinolone derivatives sometimes cause muscle, joint, or nerve issues even at clinical doses. Laboratories run long-term animal studies to flag possible carcinogenicity, reproductive toxicity, or cumulative organ impacts. These studies, trumpeted or ignored depending on the season, sketch out the risk balance required when deploying new treatments for stubborn infections. Properly conducted toxicity tests, published with full data and raw numbers, help separate sensationalism from sober risk assessment. Transparency isn’t just a regulatory box-tick — it helps every user make smarter, safer choices.
Future Prospects
Peering down the road, it’s clear the search for better, safer anti-infectives continues. Derivatives of ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate will keep showing up in patent filings and journal articles, either as direct treatments or as starting points for even bolder ideas. Many experts sense that the next breakthrough won’t come from lone inventors but from the team-up of synthetic chemists, data analysts, and clinical partners willing to share failures and successes. There’s renewed attention to balancing initial laboratory curiosity with long-term environmental and community impact, from synthesis all the way through disposal after patient use. If the last fifty years of research have taught anything, it’s that no detail proves too small when lives and ecosystems share the table with discovery and profit.
What Is It Really Used For?
Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate has a long, twisted name that won’t win any pronounciation contests, but beneath the jargon, it holds crucial value in medical chemistry. In my time working with pharmacists and researchers, this compound has shown up most often as a building block for antibiotics — specifically the ones that treat tough bacterial infections which don’t back down easily. This intermediate forms part of drugs in the fluoroquinolone family, like ciprofloxacin, which doctors prescribe for urinary tract infections, respiratory infections, and sometimes, when nothing else has worked in the hospital setting.
Fluoroquinolones came to the scene as a powerful answer to stubborn infections in the 1980s. Developing these antibiotics is never simple. Each part, each step in the recipe, needs rigor and accuracy. Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate stands out here. Back in a chemistry lab, as a graduate student, I watched people in goggles balancing on stiff stools, carefully building up the exact structure. It’s pure chemistry, with lives hanging by a thread on the other side of the process.
Why Does It Matter?
People usually see antibiotics as pills in a bottle, not as stories that start in the lab. This key intermediate helps move research from glass vials to finished medicine, connecting the science to the patient’s recovery. Without robust sources of this compound, drug companies can run into slowdowns or face issues with consistency. As bacteria keep evolving and building resistance, scientists keep tweaking fluoroquinolone molecules to stay a step ahead. Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate is right there in that process, anchoring every leap forward.
Through my own contact with supply chain discussions, the importance of quality in starting materials always comes up. Imagine nurses, doctors, and entire communities relying on drugs made from compounds that get shortchanged in purity or quality. Mistakes up front mean consequences down the line. Regulatory bodies like the FDA and European Medicines Agency have strict rules for these exact reasons. Labs keep close records, batch by batch, so every dose stacks up to the standard that patients deserve.
The Bigger Picture: Challenges and Solutions
One major concern is antibiotic resistance. It doesn’t just affect hospitals with advanced tech; it crawls into daily life, affecting anyone with an infection that simply won’t clear up. The more we lean on antibiotics, the more we need the fine building blocks like this one. Companies have to stay vigilant, investing in safer, more efficient ways to produce each intermediate without cutting corners. For example, green chemistry can reduce waste and lower environmental impact, all while keeping quality tight.
Collaborations between manufacturers, universities, and public agencies help share information and spot weaknesses in the pipeline. I’ve seen these meetings — intense, but necessary. Everyone at the table knows shortcuts don’t just ruin profits; they can cost lives. Education for prescribers and patients also plays a role: less overuse, smarter prescriptions mean antibiotics stick around longer as a tool, rather than fading into uselessness.
So, Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate isn’t in the spotlight, but it helps save lives on a scale most people never see. Behind every prescription bottle, there’s years of chemistry, strict oversight, and tough decisions about keeping everyone one step ahead of bacteria.
Understanding Chemical Formulas in Everyday Life
People often hear terms like “chemical formula” and “molecular weight” tossed around in classrooms, laboratories, or news stories about new medicines and materials. For a lot of folks, these words sound like textbook jargon, but the truth is, we all rely on an understanding of what a substance is made of, every single day. Chemical formulas tell you exactly what’s inside a compound. Imagine walking into a pharmacy: you check the packaging for ingredients, making sure what goes into your body contains what you expect. The label, showing something like C9H8O4 for aspirin, gives you a look behind the curtain. Each part of that formula makes a difference in how the compound works—and, crucially, how safe it is.
Safety and Accountability Through Exact Information
Safety comes from transparency. Think about household bleach—its formula, NaClO, isn’t just a random collection of letters and numbers. That knowledge tells factories how to produce it correctly and lets regulators check if the manufacturer follows the rules. Over the years as a science writer, I’ve seen stories where the wrong ingredient ended up in food or drugs, simply because no one double-checked the formula. It sparks recalls and lawsuits. At the root, most mix-ups grow out of ignoring what’s actually inside a product. When a company posts both formula and molecular weight, it puts itself on record: “Here’s exactly what’s in our bottle or tablet.” If every company did the same, consumers would be safer and confidence in science-backed industries would rise.
Why Molecular Weight Isn’t Just for Chemists
Most people have weighed flour or sugar at home, hoping to get a recipe just right. Chemists deal with the same thing, but the numbers are much smaller and the balance is digital. Molecular weight, measured in units called daltons or unified atomic mass units, decides how much of a substance scientists add to reaction mixtures, or how to churn out the same dosage batch after batch. This matters in fields I’ve reported on, like crop science. The right weight means a farmer isn’t spraying too much, wasting money, or too little, leaving crops exposed. Researchers use these numbers to figure out how much of a medicine reaches the bloodstream. The molecular weight also helps doctors and pharmacists understand how long a compound stays in the body or how best to combine it with other treatments.
Access to Knowledge, Not Just for Experts
These days, anybody can hop onto their phone or computer and look up what they’re taking or what’s in a cleaning product. Access to formulas and weights brings control. It gives families a chance to research products before a purchase. If a parent checks the formula of a supplement or medicine, it can shape the decision to try something new or skip it altogether. I’ve had plenty of conversations with folks who felt empowered after learning what those chemical names and numbers mean. This level of access keeps the power in the hands of those buying, not just those selling.
The Next Steps: Make Data Visible and Understandable
Manufacturers and educators have a role to play. Information about chemical makeup and molecular weight should show up on packaging, websites, safety sheets, and public databases in plain language. Schools can teach students what simple formulas really mean, not just force them to memorize. Journalists and writers have a duty to break down what these numbers mean, connecting them to real-world safety, innovation, and consumer protection. Letting everybody see and question these details will keep companies honest and products safer for everyone.
Understanding What You're Dealing With
Working with chemicals or unfamiliar substances brings its own set of risks. Getting comfortable with the material safety data sheet goes a long way. Those few pages tell you how a product can affect your body, what to avoid, and what to do if something goes wrong. Even if the product looks harmless, skin rashes, breathing problems, or fires can start from a careless mistake.
The Importance of Personal Protective Equipment
The first thing most folks reach for are gloves and goggles. That simple step has saved me many headaches. Goggles keep splashes out of your eyes, while gloves block burns or irritation. For certain powders or strong-smelling liquids, a face mask or even a respirator makes sense—breathing in just a little can leave you coughing for days.
Lab coats, full coverage clothing, and shoe covers aren’t just for show. Spilling a harsh product on your skin or getting it between your fingers can cause infections or worse. I’ve learned never to take shortcuts with gear, because nobody expects an accident until it happens.
Ventilation Matters a Lot
Cracking open windows or working beneath a fume hood often gets skipped. But one mistake with ventilation leaves fumes lingering. My own experience taught me—strong odors can cause dizziness before you know it. Setting up a fan, working outside, or using an exhaust system keeps fresh air moving and chemicals away from your lungs.
Label Everything and Tidy Up
Mix-ups have surprised even careful people. Every bottle or beaker deserves a clear label with the name of what’s inside. Too easy to forget after setting something down. After using the product, clean up right away to avoid traces getting on your hands or on someone else’s equipment. Spilled powder, drops on handles, and contaminated cloths can sneak up on you later.
Storing Products Properly
Safe storage doesn’t get enough attention. I always double-check that containers seal well, and that nothing sits near heat or sunlight unless the instructions allow for it. Some chemicals release fumes or break down if you stack them wrong or keep them in warm places. A locked cabinet keeps curious kids or pets safe, too.
Preparation for Accidents
Even routine work has surprises. Knowing the emergency exit and keeping an eyewash station or first aid box nearby can make the difference in an emergency. Washing a chemical out of your eyes or off your skin fast limits the damage. I make a habit of reading emergency instructions beforehand. It’s a small time investment that pays off under pressure.
Trust Your Instincts and Keep Learning
Working with new products, I trust my gut—if something smells strange or feels unsafe, I take a step back. If unsure, I always look up new information or ask someone more experienced. Safe habits come from listening to those caution signs inside and never assuming a shortcut will be harmless.
Staying careful protects not just you, but everyone sharing your workspace. The risks are real, but building good habits makes accidents much less likely.
The Reality of Purity in Chemicals
Anyone who’s handled laboratory chemicals knows pure compounds are more ideal than common. No manufacturer or supplier promises absolute perfection, even with a top label on the bottle. Every step—extraction, synthesis, storage, packaging—brings a chance for unwanted guests. Sometimes the impurities go undetected without thorough analysis. This can be a real headache for researchers who depend on clean materials for repeatable work.
Sourcing: Tracing Contaminants from Start to Finish
It’s easy to overlook the source. If a plant-based material is the base, fields may bring pesticide residues or heavy metals picked up from soil. For minerals, trace metals or radioactive isotopes might sneak in. Synthetics produced in industrial reactors share space with solvents and byproducts, and bits of those leftovers can stick around no matter how much washing or filtering goes on. Even the water used in processing might hand off invisible minerals or trace organic debris.
Handling and Packaging
Let’s say the compound looks pure right off the line. The problems may begin at the bottling stage. Some glass can leach sodium or silicate. Plastic containers sometimes react with the stored chemical or let in oxygen that oxidizes the sample slowly over time. Particles from gloves, labels, or even the air can fall in, especially during repackaging. A dusty storage room, humidity, or fluctuating temperatures set up a recipe for slow contamination.
Health and Functional Risks
Small impurities seem harmless on paper but aren’t easily ignored in practice. In medicines, a trace contaminant or allergen could trigger a severe reaction. Food ingredients tainted with unexpected metals or solvents bring big safety liabilities, not just recalls. Lab experiments often become unreliable: a surprising spike in a chromatography baseline or odd reading in spectroscopy can trace back to a tiny impurity, leading to wasted time chasing ghost results.
Sobering Examples from the Field
Take talcum powder and its asbestos scare, or contaminated pharmaceuticals leading to product withdrawals. Both caused damage because hidden contaminants showed up only when a crisis unfolded. These stories highlight the limits of routine screening, especially with new suppliers or unusual sources. My own work with standards has shown that trusted suppliers sometimes miss a batch, and it only comes out after repeated failed syntheses and head-scratching.
Detection and Control
Modern analysis can do a lot—HPLC, GC-MS, ICP-MS, and more tease out parts per billion. But these tools work only if labs know what to look for. Routine checks can catch common suspects, like moisture, heavy metals, or solvents. Final users also play a role; careful labeling, storage in controlled conditions, and opening containers only when necessary make a difference. Auditing supply chains and demanding transparent quality data helps, especially for high-value or sensitive applications.
Stronger Solutions for Clean Materials
Multilayered approaches keep risks in check. Sticking to trusted manufacturers with full disclosures, ordering small lots for critical jobs, and verifying each batch independently have saved my projects before. Industry groups and regulators push for stricter standards and random inspections. No system is foolproof; staying vigilant, keeping records, and following up on any anomalies gives the best shot at catching impurities before they cause real trouble.
Why Storage Conditions Shape Quality
If you’ve ever spent hours weighing and aliquoting chemicals, you know the mood in the lab can swing with the quality of your raw materials. I once watched a promising project stall because our quinolone intermediate picked up too much moisture, clumped, and, after a retest, lost more than a few percentage points of assay. Stability shouldn’t be taken for granted, especially with compounds like Ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate. This isn’t table salt—how you store it shapes every result you get.
Temperature and Light Control Matter Most
It’s tempting to tuck every bottle on the closest shelf, but experience tells another story. Fluoroquinolones react to both heat and light. Once, we left a sample exposed in a sunny room for a month, just to see what could happen. The result? The color deepened, the material failed HPLC purity, and downstream reactions collapsed. Room temperature rises above 25°C and sunlight create a perfect storm for degradation, especially when methoxy groups and fluoro substituents are in play. It makes sense to rely on cold, dark storage. In my own lab, we found the ideal spot is a refrigerator set between 2–8°C. Thick amber glass bottles go a long way to cut UV exposure, and even the tiniest ray slipping in can eat away at purity over time.
Water and Air Are Silent Spoilers
Moisture can sneak inside a poorly sealed container and start hydrolyzing esters before you notice. Years ago, we ignored a desiccant packet for a single batch and, by the following week, saw lumps and a sour smell. That batch never recovered. Desiccants aren’t just for shipping; they deserve a year-round place in your chemical cabinets. Screw-cap bottles with a soft-seal liner add protection, squeezing out the air that brings unwelcome oxygen. Oxygen can oxidize the quinolone ring, shifting purity numbers in subtle but critical ways. Regular checks with Karl Fischer or loss-on-drying methods have saved many projects in my career from expanding into full-blown recalls.
Labeling and Routine Checks Make a Difference
Small mistakes add up: skipping labels, mixing up dates, neglecting to rotate stock. Several years ago, our lab had two nearly identical containers side by side, one new, one nearly expired. A mix-up cost us weeks and delayed partner labs stuck downstream. Each container of ethyl 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate should show date received, lot number, and date opened. I’ve also found routine monthly checks—looking for clumps, color change, or cap integrity—keep surprises to a minimum.
Keeping it Safe for Today’s and Tomorrow’s Work
Sound storage isn’t about pleasing the chemical librarian or fulfilling paperwork. Protecting sample quality means teams don’t flush days’ worth of synthesis down the drain. In my experience, the best solution combines attention—cold, dry, and dark environment—with the habit of checking, labeling, and using what you have in smart order. No sophomore science course can teach the pain of a ruined experiment. But anyone who’s lost a container to the slow creep of air and light doesn’t make the same mistake twice.