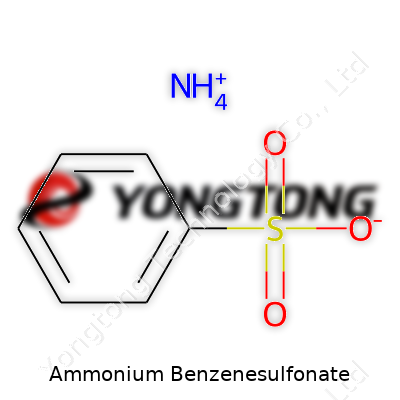
Ammonium Benzenesulfonate: A Deep Dive into Its Role, Use, and Horizon
Historical Development
Chemists started developing ammonium benzenesulfonate in the dawn of industrial organic chemistry, when industry pushed for cheaper and more reliable sulfonated aromatic compounds. This reflected a trend that moved through Europe during the mid-to-late 1800s, as chemists were hunting for water-soluble intermediates for dyestuffs and detergents. Laboratories in Germany and England experimented with sulfonation reactions, eventually leading to widespread adoption of ammonium salts due to their convenience, price, and manageable handling during scale-up. By the early 1900s, this compound found itself in textbooks, patents, and then, factories producing dyes, surfactants, and various synthetic intermediates. Each new industrial challenge demanded tweaks to purity, form, and reactivity. Because the compound linked such a broad family of aromatic sulfonates, its development reflected the broader arc of applied organic chemistry, where bench-top breakthroughs rapidly turned into practical workhorses for manufacturing.
Product Overview
Ammonium benzenesulfonate steps in as a solid, crystalline salt produced from the neutralization of benzenesulfonic acid with ammonia. Its composition (C6H5SO3NH4) grants it a place among easy-to-handle organic salts. Manufacturers value this compound for its solubility in water and its ability to act as a mild acid in solution. Real-world product forms include granules, powders, and occasionally aqueous solutions, depending on the next application or required transport safety. In factories, workers can open bags without clouds of dust, and the product stores visibly stable—no need for refrigerated warehouses or fancy dry rooms. These physical perks make ammonium benzenesulfonate preferable over some unpredictable sulfonation products. My experience watching dye and surfactant plants confirms: reliable, manageable intermediates enable round-the-clock continuity, and that's where this compound consistently delivers.
Physical and Chemical Properties
Ammonium benzenesulfonate appears as a white to off-white solid that dissolves easily in water, forming a clear and slightly acidic solution. The compound does not exhibit explosive tendencies under reasonable industrial conditions and shows little volatility. Its melting point hovers high enough that ambient conditions rarely threaten product stability. In solution, its dissociation into benzenesulfonate anion and ammonium cation leads to pH values slightly under neutral, providing mild acidic behavior. Chemically, this salt remains stable under typical storage and handling. It serves as both a source of sulfonate and, indirectly, as a weak acid or buffer component where specialized applications demand. Exposure to strong bases or acids forces decomposition into ammonia or the acid form, yet no violent or surprising behaviors occur during these common transformations.
Technical Specifications and Labeling
Producers define technical standards for ammonium benzenesulfonate by percent purity, water content, presence of unreacted ammonia, and overall color or ash residue. Most commercial stocks specify purities above 98%, complemented by statements about heavy metals or organic impurities that tail along during sulfonation. Labels list not only chemical name and formula but also hazard warnings, shelf life, solubility parameters, batch number, and recommended personal protective equipment. Regulatory documentation follows both regional and global standards, referencing the Globally Harmonized System (GHS), safety data sheets, and REACH or TSCA registrations. Reliable documentation always enables users to quickly verify identity using standard wet-chemistry or instrumental analysis methods. Often, labeling even includes recommended storage temperatures and bulk incompatibilities, helping distributors open warehouse doors without guesswork.
Preparation Method
Most industrial syntheses pursue a straightforward route: treat benzenesulfonic acid with excess ammonia or ammonium hydroxide. Experienced operators know that controlling the exotherm here prevents side reactions or loss of volatile ammonia. Like many acid-base processes, this reaction generates water, prompting distillation or drying after mixing, especially on larger scales. Equipment design leans toward high-corrosion resistance (such as glass-lined steel) because even small traces of free acid can chew through ordinary construction metals. Technicians monitor reaction pH, batch temperature, and total ammonia feed with accurate meters. Wastewater (from mother liquors or cleaning) receives neutralization—environmental scrutiny rarely takes days off in chemical manufacturing. The finished salt undergoes filtration, oven drying, sieving, and packaging using automated or semi-automated lines, which reduce operator exposure and simplify ongoing quality assurance testing.
Chemical Reactions and Modifications
Ammonium benzenesulfonate steps in as both a reagent and intermediate for a whole field of follow-on reactions. Its sulfonate group acts as a leaving group in nucleophilic substitution, especially useful for introducing more complex substituents onto aromatic rings. In aqueous or alcoholic solution, heating with alkyl halides or other electrophiles clears the way for new aryl-alkyl sulfonates. Researchers appreciate the compound’s stability during redox or condensation steps, since it does not participate in spurious side reactions. Shift to higher pH or add certain oxidants, and the benzenesulfonate core remains intact while the ammonium decomposes to volatile ammonia—a routine for transforming to sodium or potassium salts that suit other chemistry lines. In my hands, this chemical accepted chlorination or nitration without fuss, allowing for multi-gram modifications headed for dye synthesis or catalysis support applications. By tuning catalyst, pH, and temperature, labs access dozens of unique benzenesulfonate derivatives.
Synonyms and Product Names
Ammonium benzenesulfonate answers to a sprawl of synonyms, shaped by differences between laboratory, industrial, and regulatory naming conventions. Chemists in literature sometimes refer to it as ammonium benzene sulfonate or ammonium benzenesulphonate. Regional language preferences swap “sulfonate” for “sulphonate.” Marketed grades sometimes go by proprietary codes or trade designations, especially in the dye and surfactant industries. Catalogs list Chemical Abstracts Service numbers alongside synonyms and basic physical data. Navigating these names requires attentiveness, since similar compounds like sodium benzenesulfonate or potassium benzenesulfonate pop up right next to ammonium variants. Mistakes in ordering or labeling prompted several real-world inventory audits in my experience—especially when products travel across languages or regulatory jurisdictions.
Safety and Operational Standards
Anyone handling ammonium benzenesulfonate works under established protocols set by OSHA, EU-REACH, and Asian chemical management frameworks. The compound itself ranks low on acute toxicity, presenting minor irritation to skin or eyes only in concentrated form or after prolonged contact. Eye wash and emergency shower stations stand close by during scale-up and packaging. Workers don gloves, goggles, and sometimes dust masks, especially for spill cleanup or loading large hoppers. Ventilation keeps airborne dust and ammonia below threshold limits. Bulk storage sites monitor containment and corrosion, though the main risk arises from mixing with incompatible chemicals or careless neutralization of acid waste. I recall a spill response drill where operators easily swept and neutralized a few kilograms—main takeaways focused more on avoiding slips or trips in wet areas. Transporters carry documentation for compliance with road, rail, or maritime codes but face fewer hazards than with corrosives or flammables.
Application Area
Industry taps ammonium benzenesulfonate for multiple roles, starting with dye and pigment manufacturing, where it enables both direct reaction and intermediary transformations. Water-treatment products, photographic chemicals, and surfactant manufacture also benefit. In laboratory research, the compound appears as a counterion in ion exchange and chromatography work, with its pleasant handling traits and reactivity profile making it a staple. Concrete admixtures, asphalt additives, and lubricating oil blends each leverage the salt’s water-solubility or mild acidity for tuning performance metrics. In my time supporting R&D, I watched teams use the compound as a test reagent alongside more exotic sulfonates, precisely because it delivered reliable results batch after batch and allowed comparison of subtle tweaks in formulation across a dozen projects.
Research and Development
Remaining at the forefront of technical advances, ammonium benzenesulfonate attracts ongoing study. Researchers track its potential in custom organic syntheses, catalysis, battery electrolytes, and next-generation polymers. Many academic groups check whether subtle tweaks to the sulfonate environment shift activity, color, or processability for new dyes. In environmental chemistry, interest surged as alternative sulfonates fell under regulatory scrutiny for persistence and toxicity. Companies designing water purification agents and specialty surfactants regularly scan the literature for new use-cases and derivatives. Trends toward cleaner reactions, greener manufacturing, and circular economy push chemists to revisit long-standing intermediates with fresh analytical tools, and ammonium benzenesulfonate frequently appears in these investigations as a reliable benchmark or surrogate. Recent publications probe its use in solid-state electrolytes for batteries, hoping solubility and ion transport translate to cost-effective, scalable devices.
Toxicity Research
Toxicologists have cataloged the modest profile of ammonium benzenesulfonate since the early days of industrial hygiene. Most animal studies show a low risk for oral and dermal exposure under ordinary conditions. Chronic studies focused on respiratory irritation and generalized stress after repeated inhalation, revealing only mild, reversible symptoms at concentrations well above normal workplace levels. Environmental scientists monitored aquatic fate, noting the compound’s fair biodegradability and low bioaccumulation factor, so downstream impacts usually stay small. Watching regulatory updates, I’ve found authorities more worried about byproduct formation or downstream transformation (involving ammonia or benzenesulfonic acid) than primary toxicity. The compound rarely triggers concern in workplace safety meetings beyond standard good-practice reminders. Recent efforts look toward confirming results for new application areas, especially as regulations tighten for legacy sulfonate derivatives globally.
Future Prospects
Looking forward, ammonium benzenesulfonate finds itself set for stable demand in existing applications, while also drawing R&D interest for next-generation roles. The big topics in my network: cleaner synthetic routes, minimized waste or emissions, and higher utility in niche areas like battery manufacturing or green surfactant systems. Manufacturers experiment with continuous-flow processes, aiming to reduce batch-to-batch variability and environmental footprints. Academic labs sift through the fine structure of sulfonate groups, hoping to tweak properties for custom molecular electronics, catalysis, or polymer science. Industry consortia routinely swap data on additives, coformulants, and new regulatory requirements, since new environmental standards could reshape the landscape faster than any technical development. My take? As long as teams need reliable, water-soluble sulfonate intermediates, ammonium benzenesulfonate remains an integral part of both daily production and blue-sky research.
The Hidden Workhorse in Industry
Many might never come across a bottle labeled “Ammonium Benzenesulfonate” in daily life, but plenty of products depend on it somewhere along the chain. This compound pops up in places that keep things running, from manufacturing floors to the inside of batteries. If you’ve ever worked in a lab or dabbled with chemistry, you’ll know that most chemicals serve more than one purpose. Ammonium Benzenesulfonate has a few tricks up its sleeve.
Chemistry Basics: A Foundation for More
If you spend time around industrial chemistry, you know sulfonates grab attention for their ability to make things dissolve and mix that would otherwise want to stay apart. Ammonium Benzenesulfonate’s roots come from reacting aniline with sulfuric acid—transforming a common aromatic molecule into something way more useful. One never forgets the smell of sulfonates in the air during production. The mess and the energy needed reflect the strength of its chemical bonds—it’s built to last.
What Does Industry Do With It?
Plenty of uses stem from Ammonium Benzenesulfonate’s knack for breaking down barriers. In electroplating, manufacturers use it to keep metals sticking to surfaces evenly, preventing sludgy buildup and patchy finishes. That smooth surface on your phone’s chrome parts owes something to this compound. It doesn’t stop at shiny tech—companies count on its presence in dye production. Sulfonates help dyes spread smoothly on fibers, avoiding ugly blotches or color breaks. If you’ve worn a shirt dyed in bright colors, chances are chemistry like this played a role.
Another lifetime ago, I watched water treatment engineers use chemicals like this one to keep pipes and machinery free from clogs. Ammonium Benzenesulfonate works hard in detergents to keep grime and minerals from binding to critical surfaces. If you’ve seen rust on pipes or boilers, you’ll know why preventing build-up matters. Cleaner system means fewer shutdowns and lower maintenance costs. That translates into cleaner water running in cities and fewer headaches for plant operators.
In the laboratory, Ammonium Benzenesulfonate sometimes shows up as a catalyst or intermediate. Its sulfonate group brings predictable reactions, creating stable compounds. Chemists count on that stability when fine-tuning polymers and specialty chemicals.
Safety and Environmental Impact
Strong chemicals, especially those with sulfonate groups, have a reputation to watch. There are concerns about toxicity and persistence in the environment. Regulations demand tight control during use and disposal. Having worked with chemical waste management teams, you learn the hard way why rushing disposal isn’t an option. Documented spills caused lingering trouble in local streams—sulfonates don’t just vanish after a rainstorm. Plants must clean and neutralize waste before it leaves the building.
Path Toward Sustainable Use
Companies keep searching for ways to recycle and cut down on raw chemical use. Some labs experiment with biodegradable surfactants. Others recover and reuse Ammonium Benzenesulfonate rather than making more from scratch. It sounds like a small step, but multiplying these efforts across thousands of plants adds up. Real progress will depend on transparency, worker training, and public access to chemical safety records. We all benefit from cleaner, safer processes that don’t put health or water sources on the line.
Understanding the Chemical
Ammonium benzenesulfonate might show up in labs and sometimes in industrial settings. For folks who haven’t come across this before, it’s a white crystalline powder. It shows up in cleaning products, dyes, and sometimes even in research labs, so it isn’t exactly rare. Some folks see “ammonium” and shrug it off as everyday chemistry, but reading into the whole name reveals more than a simple cleaning compound. Benzene is no stranger to headlines, especially when talking about health.
Potential Hazards
Plenty of people who work with chemicals every day know you don’t take shortcuts—even if something smells or looks harmless. Ammonium benzenesulfonate can irritate skin, eyes, and your lungs if you breathe in dust. I spent two summers interning in a coatings lab, and there weren’t many days where we didn’t have to handle something that could burn if splashed or turn a cough into something worse if safety gear was ignored. Contact causes redness, sneezing isn’t rare, and nobody wants a trip to urgent care.
The sulfonate group has a knack for making chemicals more water-soluble. Washing off a spill might sound easy, but if it seeps into fabrics, you end up carrying it longer than you’d like. Some cleaning staff I spoke with described rashes after unplanned contact, because gloves seemed like a hassle for a “fast job.” It’s tempting to think one quick contact isn’t a big deal, yet repeated exposure adds up. Some data from the European Chemicals Agency notes respiratory irritation and potential digestive issues if swallowed. Not the most hazardous compound around, yet nobody should treat it carelessly.
Why Responsible Handling Matters
People value their health and want peace of mind at work. Companies don’t want injuries or long-term health complaints swirling around. Sprinkling “caution” signs everywhere isn’t enough unless the team understands real consequences. I’ve seen what happens when people get lax with chemical hygiene: ruined gloves stacked by the sink, goggles around someone’s neck, sleeves rolled up. Once our supervisor saw a few of us doing that, and work stopped until everyone geared up properly.
Data from the U.S. National Library of Medicine points out that direct contact with ammonium benzenesulfonate can aggravate pre-existing respiratory issues like asthma. Workers have sued over chronic exposure to certain sulfonates, and the settlement sums grew large. Lawsuits and insurance claims might shake the company, but the worker loses out—health doesn’t always bounce back. Knowing the chemical’s risks pushes everyone to take guidelines seriously.
Better Practices, Safer Outcomes
Simple changes help. Gloves, proper eye protection, and a working fume hood cut problems. Every chemical needs a Safety Data Sheet (SDS) on hand, not buried in a drawer. After an incident where a splash landed on someone’s arm, we updated our habit—now each step, from pouring to disposal, includes a pause for safety check. Training sticks better when real stories are shared, instead of just reading bullet points off a slideshow.
Engineering controls—such as scrubbers and closed handling systems—keep the air and space clean. Rushed work causes shortcuts, and that’s when accidents happen. Shifts run smoother and everyone makes it home healthy. I learned in my own work that speaking up about broken goggles or cracked gloves doesn’t slow us down; it sets an example that health comes first.
Health and chemical safety aren’t only about strict rules. They’re about building habits and sharing responsibility. Handling ammonium benzenesulfonate safely means caring about your team and choosing a workplace where people watch out for one another.
The Basics of Ammonium Benzenesulfonate
Ammonium benzenesulfonate carries the formula C6H5SO3NH4. You’ll find one ammonium ion (NH4+) paired with a benzenesulfonate ion (C6H5SO3-). This pairing isn’t just about balancing charge; it means the union of a common organic ring, benzene, and a familiar ammonium group. Neither are rare in the world of chemistry, but together, they show up in industries such as dyes, pharmaceuticals, and analytical chemistry.
Why Knowing the Formula Matters
Back in school, many kids see these formulas on the whiteboard and wonder what difference it makes. I remember that feeling in my first chemistry lab, hunched over flasks and pipettes, trying to keep track of cations and anions. People who work with specialty chemicals don’t just memorize the name—they map out the formula because one change in an atom or an ion leads to different reactivity, safety limits, and application potential.
Miss the ammonium ion? You’re left with sodium benzenesulfonate or even the free acid. Plug in the wrong formula when ordering or mixing in a lab, and nothing turns out as planned. So the formula isn’t just trivia—it’s the gateway to understanding what to expect from a chemical during storage, shipment, or reaction. Chemical data sheets always begin with the formula for this reason.
The Role in Real-World Chemistry
Ammonium benzenesulfonate pops up during sulfonation processes, where benzene meets sulfuric acid, forming benzenesulfonic acid. Toss in ammonium hydroxide, and ammonium benzenesulfonate takes shape. Through this method, the chemistry world found a route to water-soluble salts needed for modern organic synthesis and dye manufacture.
Sulfonate groups are known for boosting water solubility. The ammonium ion, unlike sodium or potassium, can bring specific reactivity in synthesis. Years back, I worked with salts like this to carry organic parts into aqueous solutions—a tricky task otherwise. The formula gives the molecular mass needed for precise measurements, plus it predicts how much can dissolve at a given temperature.
Health, Safety, and Regulatory Needs
Handling chemicals with clear formulas cuts down on risk. Ammonium salts can release ammonia under certain conditions, especially if storage isn’t sealed tight. Reading the formula lets you check for hazards. You spot the ammonium, expect a need for good ventilation, and avoid strong acids that could set off a reaction.
Regulators rely on clear formulas when setting workplace limits or flagging possible water contaminants. The sulfonate part signals potential concern for aquatic life, and the ammonium raises questions about nitrogen pollution. In practice, clear labeling based on formula supports everyone’s safety—from plant technicians to lab workers.
Steps Toward Solutions
Training in basic chemistry, especially the writing and reading of chemical formulas, gives workers tools to make safer choices. In my own experience, companies with regular training sessions see fewer mistakes in the lab. Software tools that let people search chemicals by formula or structure also help cut down on ordering mishaps and accidental substitutions.
Manufacturers and users benefit by publishing formulas on material safety data sheets and labels. With C6H5SO3NH4 right there, there’s no guessing or confusion—and chemistry, at its core, depends on accuracy.
Understanding the Chemical’s Nature Helps Everyone
Ammonium benzenesulfonate usually turns up in the laboratory or production line as a white solid. Being around industrial chemicals for years, I’ve learned that the label doesn’t tell the whole story. This one holds both the risks of incompatible mixing and the potential to release irritating fumes if it’s not kept in check. The need for a practical approach to its storage stands out—not just because safety manuals say so, but also because accidents have real-life costs. People, equipment, and business rely on common sense and structure in chemical handling.
Separation Cuts Down Risk
Over the years, I’ve seen what happens when chemicals mix unintentionally—leaks, reactions, panicked cleanup. Storing ammonium benzenesulfonate away from strong oxidizers or acids isn’t a hint from a textbook; it’s a crucial step learned from mistakes. Corrosive acids like hydrochloric or sulfuric can start a reaction that nobody wants. Space out incompatible materials, and label each zone clearly—this reduces slip-ups and makes fast checks possible during audits or emergencies.
Keep It Dry and Ventilated
Humidity creates headaches. Anyone who’s opened a leaky container knows how a powder turns clumpy or sticky—then the trouble multiplies. Moist ammonium benzenesulfonate can release hazardous fumes. Good ventilation flushes out anything that escapes. I always recommend checking for water damage in storage rooms and fixing leaks right away. Plastic liners, desiccants, or climate control stop moisture in its tracks. All of these beat chipping away at solidified lumps, which cost time and expose workers to airborne dust.
Labeling and Unopened Packaging Make a Difference
Labeling each drum or bag pays off over the long haul. Staff turnover happens, memory fades, but clear marks guide even the newest hires. From my experience, always store chemicals in their original, sealed containers if possible. Cutting corners by pouring leftovers into random jars invites confusion. Damaged packaging deserves a closer look. A split bag might not seem urgent, yet over time, a slow trickle can build up residue, cause slips, or create a mystery chemical heap for the night shift.
Temperature Control Prevents Surprises
Factories and storerooms don’t always have perfect climate control, but extreme heat or cold should be avoided where possible. High temperatures can hasten chemical breakdown or gas release. If direct sunlight hits a stack of drums day in and day out, you’re running a risk. Find a shaded, stable area if temperature-controlled rooms are unavailable. Use simple monitoring devices. A cheap thermometer or temperature log gives warning signs early—tight budgets don’t excuse neglect.
Training and Emergency Planning Complete the Picture
The best routines fall apart if people skip training. Regularly walking through chemical storage guidelines as a team keeps safety fresh. I run short safety drills every few months and post emergency contacts in plain view. Accidents rarely follow a schedule, and readiness matters more than policy binders. Quick response relies on knowing where safety showers, eyewash stations, and spill kits are located.
Building a Culture of Care
Safe storage isn’t only about following laws or ticking boxes for inspectors. It’s about protecting health, property, and the people we work with every day. By respecting the risks and putting clear steps in place, handling ammonium benzenesulfonate becomes less about fear and more about responsibility.
Understanding Exposure: Where Hazards Lurk
Ammonium benzenesulfonate often comes up in discussions around industrial cleaning agents and dye production. For anyone working around this chemical, it means dealing with white or off-white crystals that dissolve easily in water. Chemical plants, textile factories, and even some research labs keep this compound on hand because of its surfactant properties. In my time spent consulting at an industrial facility, safety meetings always circled around the possible harm from routine handling of substances like this.
Pathways to Health Risks
One big concern pops up during inhalation and skin contact. Ammonium benzenesulfonate releases irritating fumes, especially if heated or mixed with acids. Just a brief whiff during a chemical spill makes your throat and nose burn, while skin contact often leads to rashes or mild burns. Long-term exposure remains more troubling. Some animal studies link benzenesulfonates to cell inflammation after repeated doses.
Human cases reported in the chemical manufacturing world often involve minor respiratory and dermal symptoms, but these incidents highlight a larger issue: many workers forget how even moderate exposure stacks up over months or years. My own days near filling machines gave me daily reminders of how accidental splashes can sting and itch well beyond the workday.
Impact on the Environment
Beyond direct human hazards, ammonium benzenesulfonate spills can bring trouble to river and lake ecosystems. The compound shows moderate to high solubility, so it spreads quickly through water systems. Fish and invertebrate studies reveal reduced growth and reproduction at surprisingly low concentrations—data from Europe even triggered local water advisories after accidental discharges from dye plants. Watching news coverage about these fish kills, you realize the extent a single factory leak can impact both natural food chains and nearby communities.
Industrial Fire and Chemical Reactions
Fire presents another layer of risk. Ammonium benzenesulfonate rarely explodes on its own, yet it produces toxic gases like sulfur oxides and nitrogen compounds if caught in a blaze. These byproducts not only complicate firefighting but also lead to large-scale evacuations. A major warehouse accident in southern China forced entire neighborhoods to clear out because of the smoke generated by a chemical pallet fire. The images of yellowish clouds above city blocks serve as cautionary tales for fire prevention protocols.
Practical Solutions: Reducing the Dangers
The path forward relies on smarter engineering and well-trained personnel. Installing chemical fume hoods and local exhaust systems cuts indoor air contamination nearly in half, based on figures from the American Industrial Hygiene Association. Personal protective gear—gloves, face shields, sturdy aprons—needs regular checks for tears and chemical residue. Regular medical screenings for anyone exposed on a daily basis also offer an early warning system for skin and lung irritation.
Responsible companies invest in spill response kits and secondary containment barriers as added layers of security. Fast action during leaks can spare workers and neighbors from serious harm, as I once saw when a two-person cleanup team contained a bench-top spill in under five minutes—training made the real difference that day.
On the regulatory side, adjusting discharge permits and enforcing stricter limits on waterway releases can force better stewardship over local environments. Transparent reporting of workplace exposure numbers gives researchers more to work with, building a stronger understanding for future efforts at risk reduction.
Real progress comes from treating these hazards as shared problems, not just technical hurdles. Every safeguard, from better ventilation to cleaner handling routines, protects both workers' health and the surrounding ecosystem. Personal vigilance goes a long way, but collective responsibility shapes safer outcomes for everyone in the long run.