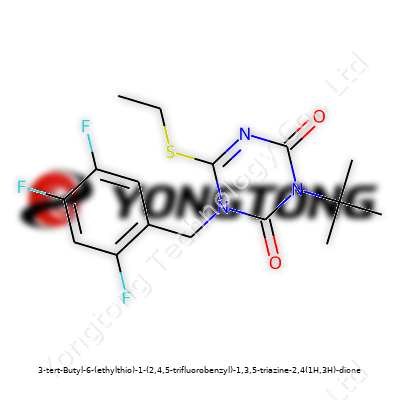
3-tert-Butyl-6-(ethylthio)-1-(2,4,5-trifluorobenzyl)-1,3,5-triazine-2,4(1H,3H)-dione: A Closer Look
Historical Development
Chemists began exploring triazine analogs in the mid-20th century, originally searching for novel herbicides and pharmaceuticals. Over the decades, researchers tried out various substitutions, landing on the tert-butyl, ethylthio, and trifluorobenzyl configurations for their unique combination of stability and reactivity. The specific compound—3-tert-butyl-6-(ethylthio)-1-(2,4,5-trifluorobenzyl)-1,3,5-triazine-2,4(1H,3H)-dione—builds on many years of iterative synthesis, whenever advances in fluorine chemistry or organic synthesis provided new building blocks. Modern discovery owes a lot to the work in selective fluorination, which enabled chemists to try out the 2,4,5-trifluorobenzyl group, adding to the molecule's bioactivity and environmental resilience.
Product Overview
People often turn to this compound thanks to its multi-functional triazine core and combination of bulky and electron-withdrawing side groups. This structure gives the molecule not only a foothold in the agrochemical space, where persistence and activity matter, but also places it on the radar for pharmaceutical leads and specialty resin additives. Triazines like this one show up anywhere the balance of reactivity, stability, and functional versatility is valued—for instance, as intermediates in step-by-step syntheses or as scaffolds in medicinal chemistry.
Physical & Chemical Properties
3-tert-Butyl-6-(ethylthio)-1-(2,4,5-trifluorobenzyl)-1,3,5-triazine-2,4(1H,3H)-dione typically comes as a solid crystalline material, most often a pale or off-white powder. It shows poor solubility in water, a direct result of its substantial nonpolar groups and fluorinated ring. The compound melts at a relatively high temperature, indicating solid crystal lattice energy. Exposure to sunlight or elevated temperatures does little to break it down, due to the electron-withdrawing effect of the trifluorobenzyl moiety and the shielding from bulky tert-butyl groups. Its chemical stability means it sits comfortably in storage under nitrogen or argon, without unexpected decomposition.
Technical Specifications & Labeling
A product label usually details purity, batch number, and any residual solvent levels to flag handling precautions. Purity often stays in the 98-99% range, with traces of synthesis byproducts kept below 1% weight. Material safety data sheets focus on inhalation and direct contact precautions because even stable solids can generate fine dust or react at elevated temperatures. Labelling requirements differ depending on the intended application, but professional suppliers always include clear hazard pictograms and recommended handling instructions, along with a certificate of analysis to ensure reproducibility in research or industrial settings.
Preparation Method
Synthesizing this compound starts with assembling the triazine-2,4-dione core. Most routes begin by reacting cyanuric chloride with the appropriate alcohol or thiol, then introducing tert-butyl and trifluorobenzyl groups in controlled steps. For the ethylthio substitution, early-stage reaction with ethanethiol under basic or acidic conditions works well. The trifluorobenzyl group is added through nucleophilic substitution, often following improvements in palladium-catalyzed couplings. The reaction produces a mix of intermediates, requiring chromatography or crystallization to isolate the final product. Optimizing reaction temperature and solvent selection remains critical, since trace moisture or impurities could lower yield or affect the end-product’s physical consistency.
Chemical Reactions & Modifications
The molecule’s structure allows selective reactivity without throwing away overall stability. Chemists can further modify the ethylthio or tert-butyl groups through oxidation, substitution, or even click chemistry–making the compound a useful synthetic building block. The electron-poor triazine ring resists attack by bases but can be coaxed into selective ring-opening reactions under acidic or reductive conditions. This ability to add or swap groups without breaking the main ring opens doors to analog design in search of more active or less toxic variants. Research labs use it to test modifications that may lead to stronger interaction with biological targets or greater environmental persistence, aiming to fine-tune its performance for specific end uses.
Synonyms & Product Names
You might come across this molecule under a range of alternative names, depending on the supplier or context. Shortened forms like 3-tert-butyl-6-ethylthio-1-trifluorobenzyltriazine-dione sometimes show up in research literature. Internal company codes like TBT-ET-TFBZ or catalog numbers provide further reference points, especially for inventory and import-export documentation. Having reliable synonyms listed on documentation reduces confusion in procurement and regulatory approvals, especially with triazine derivatives, where one minor change in the side chain turns it into a whole different chemical with its own hazards and uses.
Safety & Operational Standards
Anyone handling this material in a lab or production setting pays close attention to exposure controls. Working with solid-phase triazines, people rely on gloves, goggles, and, when working on scale, fitted respirators, especially during weighing or transfer steps. Dust control and proper ventilation matter, since fine particles can irritate eyes and airways. Disposal always follows local and international regulations for halogenated organics, since fluorinated rings pose a problem for environmental breakdown. Routine training and well-tested procedures help head off accidental exposure or unplanned chemical reactions, safeguarding personnel and the surrounding environment.
Application Area
Products in this family often pull double duty as herbicidal agents—taking advantage of both their activity spectrum and environmental persistence—and as custom intermediates for complex pharmaceutical syntheses. Specialty polymer manufacturers investigate its use as a crosslinker or stabilizer thanks to its resistance to UV and thermal degradation. Ink and dye industries seek out triazine cores for their colorfast properties and compatibility with modified resins. The breadth of use cases comes down to the molecule’s backbone, which combines robust chemical stability with flexibility in further modification, allowing tailored performance for agriculture, healthcare, polymers, and beyond.
Research & Development
R&D teams experiment with analogs using the same backbone but altered side chains, hunting for better performance or safety in agrochemicals and drugs. Partnerships between industry and academia contribute to understanding how structural tweaks influence biological activity, solubility, and resistance to metabolic breakdown. Computational modeling now plays a pivotal role, letting researchers simulate how slight changes affect binding affinity or toxicity before moving to the bench. Newer synthetic routes look for greener processes, reducing waste and hazardous reagents while improving atom economy. As restrictions on persistent organic pollutants get tighter, every new version faces more scrutiny for unintended effects.
Toxicity Research
Toxicologists keep a close watch on compounds containing both halogenated benzyl rings and triazine diones. Studies suggest these structures resist breakdown, persisting in soil or water long after application, raising concerns about bioaccumulation. Lab trials on rodents and aquatic organisms measure acute and chronic effects, helping set exposure limits and recommended handling protocols. Data so far show a low acute toxicity profile but highlight risks connected to repeated or large-scale exposure. Long-term monitoring in agricultural runoff, combined with modeling potential metabolic products, feeds into updated guidelines for responsible use.
Future Prospects
Over the next several years, demand for high-performance chemical building blocks will likely grow, especially those that offer both effectiveness and resilience against environmental or biological attack. Whether regulation tightens on persistent chemical residues or grows more favorable thanks to improved molecular design, triazine derivatives that combine effective action with reduced environmental footprint stand to gain wider adoption. Research focuses on developing analogs easier to break down after use, or that express activity only in targeted scenarios, making agricultural and medical applications safer for both workers and end users. Advances in fluorine chemistry and green synthesis could set new standards for what these compounds deliver, both in the lab and on the field.
Why This Chemical Draws Attention
People who work in agriculture or environmental science hear about active ingredients that control weeds. This compound enters the conversation because of its role as a herbicide. Triazine-based chemicals, like this one, have shaped how farmers tackle tough weeds that threaten yields. In fields where broadleaf weeds overrun crops, farmers count on targeted chemicals for reliable suppression. Without tools like this, corn and cereal crops would face steeper losses and higher labor costs.
How It Shapes Modern Weed Control
Chemicals in this family lend themselves to pre- and post-emergence weed control. Farmers spray these solutions to stop weed seedlings from growing, protecting young crops as they start their life cycle. I spent summers on family farms watching how weed outbreaks force growers to gamble: spray early and risk using too much, or hold back and risk a weed explosion. The right active agent lets them strike a balance, protecting both the crop and the environment.
This triazine derivative blocks photosynthesis in unwanted plants. By targeting specific enzymes, it keeps weeds from converting sunlight into usable energy, slowly starving them. Decades of science anchor this process. Studies from agricultural universities show how triazine herbicides work in even tough growing conditions. Corn and sorghum fields in the U.S. Midwest often rely on these compounds to stay ahead of Palmer amaranth or waterhemp infestations.
Serious Issues Drive Debate
Weed resistance stands as a growing challenge. Many farms use the same chemicals year after year, and resistant weed populations build up when there’s little rotation between herbicides. My peers and I swap stories at local co-ops about those first signs of failure—patches of weeds that survived spraying, cleaning out combines clogged with stubborn green stalks. Figures from the Weed Science Society of America show that herbicide resistance spreads in fields across the country, raising the cost and effort required to keep fields productive.
Triazine compounds also tail concerns about human health and water safety. Groundwater monitoring projects, like those led by the U.S. Geological Survey, detect triazine residues in some rural wells. Long exposure links to health risks, including hormone disruption. This drives scrutiny from regulators who balance the needs of food production with the right to clean water.
Seeking Practical Solutions
Researchers call for rotating herbicide classes. Integrated weed management helps—farmers use crop rotation, mechanical cultivation, and multiple chemical modes of action to keep weeds off balance. Some companies fund next-generation chemistries that claim to hit weeds in new ways. Extension agents run workshops showing how to spot resistance early, teaching techniques that help farmers cut back on chemical use.
I have watched local growers sharpen their spray programs, track weather conditions, and reduce overlap. Technology such as GPS-guided sprayers, field mapping, and variable-rate application makes this practical. Policies that encourage safe chemical handling and transparent reporting support trust between farmers, regulators, and communities. Progress in this area means everyone plays a role—farmers, scientists, public health professionals, and consumers.
Why It Matters Now
As world food demand rises and climate shifts bring new weed pressures, the role of specialized herbicides like this one remains a hot topic. Farmers face choices daily—selecting chemistry that manages weeds, ensures crop safety, and avoids long-term harm to public health and the environment. Looking at real farm life and data from ongoing research shows that solutions must blend practical know-how with responsible science.
Practical Lessons From Handling Hazardous Compounds
Working in a lab, the rules for handling any chemical aren’t just official paperwork—they become muscle memory after a few close calls. There’s nothing theoretical about a splash that stings or a fume that catches in your nose. Over the years, strong respect for basic safety gear set in. Wearing gloves, goggles, and a lab coat saves fingers and eyes more times than anyone cares to admit. Plenty of people skip these steps, thinking “just a quick transfer” won’t hurt. That’s the kind of shortcut that puts someone in the ER.
Knowing What You’re Up Against
Every chemical label tells a story. If you don’t know what you’re working with, you’re in the dark. The material safety data sheet reads boring but breaks down real risks, like how a chemical acts with water, air, or any common material in the area. Some substances explode with a little heat; others turn caustic after mixing. Once watched a bottle of concentrated acid eat through a glove and start smoking. After that, double-checking storage instructions stopped feeling optional.
Good Storage Practices Make All the Difference
Chemicals aren’t meant to mix with whatever’s sitting on the next shelf. Strong acids need their own space, away from strong bases or solvents. Labels face out, lids tight, never stacked on shaky shelves. A sloppy storage area turns a small spill into a big cleanup fast. The old-timers get this: organize everything, know what’s beneath, and never store bottles above eye level. It only takes one dropped container to spread panic.
Working With Proper Ventilation
Strong smells signal more than inconvenience. Plenty of compounds let off fumes that harm lungs or cause dizziness in minutes. Fume hoods give a controlled highway out, not just a fancy cabinet to lean over. Turning on the vent and keeping your face out of the line of fire trumps convenience. Once had a co-worker pass out because a hood stayed shut off for a five-minute task. No quick job is worth that risk.
Clean Up Right Away
Spills happen—how someone reacts makes all the difference. In school, leaving a small chemical spot on the counter felt harmless. After watching instructors react fast with spill kits and neutralizing agents, the lesson stuck. Immediate cleanups with dedicated tools keep incidents minor. Rags from home or shared bathroom towels spread trouble. Responding with the right attitude and gear reduces long-term hazards for everyone.
No Solo Experiments
Working alone with reactive or corrosive substances invites disaster. Emergencies need fast reactions—knowing someone’s five rooms away can turn minor burns or splashes into life-changing injuries. On one occasion, a lab mate’s watchful eye prevented a small slip-up from growing ugly. Spaces that encourage a “buddy system” cut risks more than any poster on the wall.
Keep First Aid and Emergency Gear On Standby
Routines change. Labs get busy, or new people join who miss old warnings. Eyewash stations, showers, and fire extinguishers must stay ready, not blocked by boxes or old equipment. Even seasoned experts freeze up in a crisis if they don’t know where to run. Drills, though tedious, map out escape plans in stressful moments.
Continuous Learning and Culture Matter
People can memorize rules, but culture in a lab or workshop keeps those rules alive. Sharing stories, even the embarrassing ones, helps new folks learn hard lessons the easy way. Training never ends, because chemicals and methods keep evolving. Building a team that talks openly about mistakes and learns together builds a safe place to work.
Why Stability Shapes Real-World Choices
Chemical stability isn’t just a technical box to tick—it's a daily concern for many people working with any kind of chemical product. If you’ve ever opened a jar in your garage after a year or found a bottle in your lab that’s gone yellow, you’ve stared stability in the face. The shelf life of a product doesn’t come down to luck. It comes down to design, testing, and respect for the science behind what we keep on our shelves.
Let's look at the facts. Many chemicals degrade with moisture, exposure to light, or temperature swings. Some react slowly and harmlessly, but others can change completely or even create hazards if ignored. Manufacturers bear the responsibility for guiding users, offering clear information on what keeps each product safe and effective. The safety data sheet should never gather dust—it contains advice shaped by controlled studies and real-world incidents alike.
Getting Storage Right: Lessons Learned
I’ve kept everything from salt solutions to industrial bricks of acid in my own work. Dryness is crucial for many powders and granules; cap left loose and you’ll often see clumps or color changes. Keeping bottles closed, using desiccants, or choosing ventilated but dry spaces makes all the difference. Some chemicals—think peroxides or photosensitive dyes—break down fast with sunlight. Amber glass comes in handy here, but so does simply keeping containers in the dark.
Hazards can build when simple rules go ignored. College labs carry horror stories about corroded containers or pressure build-up from forgotten bottles, particularly with reactive or volatile substances. Labeling every container, recording dates of opening, and separating incompatibles cannot be skipped. These habits aren’t only about following orders—they’re built over decades of lessons, accidents, and near misses.
Why Guidelines Can't Be Generic
No two products need the same approach. Water-based cleaners often last for years below 25°C, so sticking them in a hot attic shortens their life, while some solvents break down only at much higher temperatures or if mixed with other chemicals. I've seen food additives lose flavor or function after a few summer months even if “safe” by the numbers.
Stability studies can’t control your climate, but it's clear that cool, dry, and protected places slow chemical reactions. Monitoring humidity, using climate-controlled storage, or refrigerating sensitive compounds isn’t overkill. In fact, it’s how major research labs avoid costly mistakes and lost work.
What Works for the Big and Small Player
Clear labeling, rotation of stock, and respecting storage guidelines keep businesses efficient and safe. Pharmaceutical manufacturers, for example, must guarantee their drugs won’t degrade below standards. That means not just picking the right preservative but designing packaging to limit oxygen, moisture, or light. Even small shops and home users benefit from similar steps, if only to avoid wasted money or accidental hazards.
A little extra care, along with honest guidance based on good science, keeps chemical products stable and safe through the real demands of daily life. The lessons are practical, and the stakes can be high—including health, safety, and cost. What we store and how we store it deserves respect grounded in real evidence and remembered experience.
Solubility Shapes More Than Just Chemistry Labs
People usually stumble across questions about solubility in class or on a safety sheet at work. For chemists, solubility often decides which method actually works and which ideas stay on paper. Shoving a compound into water and watching it disappear—or just sit there—ends up telling a lot more about those molecules than you might expect.
Sodium chloride blends into water in seconds. Table salt’s atomic structure splits apart between sodium and chloride ions, and water molecules swarm in to trap those charged bits. It works because opposites attract. Water’s a polar molecule: its ends carry small charges. Ions love that sort of thing, so mixing table salt into water almost feels inevitable. The result: saltwater that keeps you hydrated or preserves food.
Oil and Water: A Story Told By Structure
Step outside salt and you run into compounds like oils. These mingle with gasoline or alcohol, but pour some olive oil into a glass of water, and both sides keep apart. This happens for a reason. Hydrocarbon chains in oils don’t carry charges, and they hate polar environments. Water stays with water; oil clings to oil. This is why oil spills spread on lakes rather than vanishing. Cleanup crews rely on this trick, using materials that absorb oil but ignore water.
I’ve seen people struggle with simple things—trying to wash sticky glue or permanent marker off skin with water, not realizing that you need rubbing alcohol. Anything nonpolar likes nonpolar solvents. A rule: like dissolves like. Predicting which compound breaks up in water becomes a guessing game unless you know what’s inside. Read the label; check for ions or charged groups if possible. Sometimes, even acidity or pH pushes a borderline compound into solution.
Why Solubility Challenges Matter in Real Life
The stakes on solubility stretch into medicine. Doctors can’t use many drugs unless those drugs dissolve in blood or other fluids. Drug researchers chase ways to tweak molecules, adding a salt group here or a small side chain there to flip a stubborn compound from useless to lifesaving. Take ibuprofen—raw ibuprofen prefers oil, not water. Scientists change its structure to form a salt, making it dissolve fast enough to work inside the body.
Water purity relies on solubility, too. Pollutants in groundwater either spread out or stick together based on which solvents they favor. Farmers and engineers have to worry about what happens after fertilizers meet rainwater. If a pesticide sticks to the soil, that’s better for crops, but some chemicals cut loose and flow into rivers. Tracking these pathways relies on knowing solubility by heart, not just trusting a manufacturer’s chart.
How to Test: More Than Just Guesswork
Lab techs and hobbyists, myself included, sometimes go old school. Take a pinch of the stuff, drop it into a tube of solvent, shake, and see what happens. Cloudy solution? It’s mixing. Crystals at the bottom? No dice. But this approach isn’t always safe. For strange compounds or anything with a hazard sign, check published studies or consult chemical suppliers. Most reputable sources publish water solubility, but don’t ignore peer-reviewed research or field data.
Everybody gets curious about what dissolves where, because it patches together lab safety, medicine, pollution, and everyday chores. Taking shortcuts or blind guesses risks more than just wasted time; sometimes it risks your health or the environment. Understanding the basics—how charge, structure, and solvent fit together—helps turn solubility from a quiz question into a practical skill.
Daily Life Meets Chemical Risk
My earliest memory of handling chemicals came from painting the fence with my father. We didn’t use gloves. The next day, red itchy bumps showed up on my hands. I didn’t think much of it then, but over time, I learned these small exposures build up. The body reacts, sometimes in ways that don’t show right away—like headaches that won’t let up, or a tightness in the chest that keeps you up at night. Hazard labels aren’t just there for show; contact with strong cleaners, pesticides, or industrial solvents brings real risk.
Short-Term Hazards Add Up
Direct exposure can kick off a range of health problems. Touching some chemicals may cause rashes or burns—simple soap and water won’t always fix it. Breathing fumes might spark asthma attacks or dizziness. Some chemicals go after the eyes or nose, causing irritation within minutes. Every year, emergency rooms see people with chemical-related eye injuries or breathing problems, often from everyday products. According to the American Association of Poison Control Centers, millions call for help related to chemical exposure. That adds cost, pain, and sometimes lasting health concerns.
Long-Term Effects Stick Around
Continual low-level exposure creates problems that don’t always scream for attention right away. Chemical workers and even farmers sometimes find out years later about issues like nerve damage, hormone imbalances, or trouble concentrating. I have a friend who ran a small dry-cleaning shop. She worked there for over a decade and eventually developed breathing difficulties and skin sensitivities. Doctors linked her problems to regular inhalation of cleaning solvents. Studies support these stories—repeated workplace exposure to perchloroethylene and formaldehyde can raise cancer risk, even for people who never smoked a day in their life.
Safety Gaps at Home and Work
Most folks don’t think twice about pouring a drain cleaner, but labels warn for a reason. Teams at the Environmental Protection Agency cite thousands of accidental exposures in homes each year, especially among young kids. Occupational Safety and Health Administration statistics highlight that many workplace injuries stem from ignored safety gear or missing ventilation. I’ve seen busy small businesses skip gloves or masks because they felt awkward or slowed the work. That’s exactly when trouble finds people.
Ways to Protect Yourself and Others
Staying safe isn’t just about reading the label and moving on. Smart habits matter—wearing protective clothing and working in well-ventilated spaces cuts down on risk. Training makes a difference too. Many companies offer short safety talks that explain what those warning symbols really mean, why gloves matter, or how to handle spills without panic. The CDC gives easy-to-follow guides for storing and disposing of chemicals at home: keep products in original containers, out of reach from kids, and never mix cleaners.
Technology also helps. Modern sensors can alert workers to gas leaks nobody would notice otherwise, and lockable cabinets keep hazardous products away from curious hands. Still, nothing beats watching out for your own health and reacting early when something seems off—be it a funny smell, unexplained rash, or just feeling dizzy at work.