6-Chloroindolin-2-one: Making Sense of a Key Chemical Building Block
Historical Development
Chemistry covers years of trial and error, building on what others leave behind. 6-Chloroindolin-2-one, also called 6-chlorooxindole, first showed up in the mid-20th century, as pharmaceutical research hunted for fresh molecules to fight illness. Scientists drew inspiration from oxindole frameworks showing up in plant metabolites and early medicines. They began adding halogens like chlorine to the indolin-2-one skeleton, searching for new properties, therapies, and drug ideas. Older patents and journals reveal medicinal chemistry groups turning out libraries of these analogues, hoping to strike gold amid all that lab work. Today, 6-chloroindolin-2-one’s roots tie tightly to that wave of innovation, as research groups in Asia, Europe, the United States, and elsewhere kept broadening its reach from basic chemistry to materials development, crop protection, and new drug candidates.
Product Overview
This white to light beige solid reflects a small tweak to an ancient core structure. Its molecular formula, C8H6ClNO, puts a chlorine atom on the indolin-2-one ring system, swapping in new reactivity compared to its parent molecules. Labs buy this compound by the gram or kilogram, using it as a research standard or chemical stepping stone for bigger, more complex targets. Many vendors ship tightly sealed bottles with traceability and purity documentation.
Physical & Chemical Properties
6-Chloroindolin-2-one melts around 195–197 °C, and it keeps a fairly stable solid form under typical lab storage. The compound does not dissolve much in water, turning instead to classic organic solvents like chloroform, methanol, or DMSO, depending on the needs of a specific synthesis. Its aromatic core and carbonyl group make it a good candidate for both nucleophilic and electrophilic reactions, and that chlorine at position-6 adds a dash of electron-withdrawing effect, guiding reactivity and selectivity in certain organic transformations. From the nitty-gritty of its NMR spectra to UV-vis absorption features, the molecule comes well-mapped, letting chemists confirm its identity and purity with modern instruments.
Technical Specifications & Labeling
Producers of 6-chloroindolin-2-one apply strict labeling and traceability to every order, since chemists don’t want surprises in their starting materials. Purity often sits at 97% or higher according to HPLC or GC data, with trace impurities and residual solvents listed right on the certificate of analysis. Labs expect detailed batch numbers, storage recommendations—keep it away from direct light, store cool and dry—plus full hazard classification under GHS or local chemical regulations. The use of unmistakable, legible hazard signs speaks to broad adoption of chemical safety, ensuring anyone handling this compound knows the risks associated with inhalation, ingestion, or skin contact.
Preparation Method
Synthesis teams turn to several reliable methods to make 6-chloroindolin-2-one. One mainstay involves the chlorination of indolin-2-one itself by using agents like N-chlorosuccinimide (NCS) in a controlled organic solvent, then isolating the specific 6-position isomer through careful separation and purification steps. Other approaches use advanced cross-coupling chemistry or even high-temperature cyclization of precursors bearing a pre-installed chlorine, depending on cost, throughput, and green chemistry preferences. Industrial outfits may run these reactions in reactors under inert gases, with full waste treatment and emissions controls to limit environmental footprints. Academic groups still hunt for more efficient and eco-friendly routes, tacking on microwave, enzymatic, or flow chemistry tricks to get the cleanest product at scale.
Chemical Reactions & Modifications
6-Chloroindolin-2-one acts as a versatile platform for further molecular editing. The chlorine atom allows selective substitution through nucleophilic aromatic substitution, opening a path to a range of new compounds with varied utility. The NH group and nearby ketone can interact in classic acylation, alkylation, amination, and condensation reactions useful for tailoring molecules aimed at new drugs, dyes, and agrochemicals. Its aromaticity and electron distribution set the stage for advanced coupling reactions, letting chemists build up larger architectures based on the oxindole motif. In hands-on research, 6-chloroindolin-2-one often becomes a bridge compound, taking simple starting materials to complex, bioactive frameworks in just a few steps.
Synonyms & Product Names
Lab catalogs and research papers usually call it 6-chloroindolin-2-one, but the set of synonyms shows its rich chemistry heritage. Terms like 6-chloro-2-oxindole, 6-chloroindole-2-one, or CAS number 21218-53-1 keep cropping up, while some suppliers might refer to it as 6-chlorooxindole for short. Comprehensive product sheets clarify all identifiers, ensuring researchers don’t mix it up with similar compounds.
Safety & Operational Standards
This molecule fits within the broader class of lab-controlled chemicals, bringing an expectation for gloves, goggles, and local exhaust ventilation. It can cause irritation to skin, eyes, and mucous membranes, and vapor or dust may present inhalation hazards if handled without proper controls. Official hazard labeling follows local and international laws, and chemical hygiene plans advise immediate cleaning of spills, safe storage away from incompatible reagents, and documented procedures for disposal. Emergency response teams prepare for accidental exposure through eyewash stations, safety showers, and air monitoring, reflecting decades of lessons in chemical safety and accident prevention. Real-world experience shows that these protocols not only protect workers but also maintain research quality by reducing sample contamination and cross-reactions.
Application Area
6-Chloroindolin-2-one’s main home sits squarely in the research bench or pharmaceutical lab. Drug discovery teams use derivatives of this scaffold to chase down new inhibitors for targets like kinases, serotonin receptors, and proteases. Agrochemical developers try out its analogues in search of crop protection agents or growth regulators. A few teams studying specialty dyes and organic electronics have built small test sets around oxindole cores, exploring how halogenation tweaks material properties. As a chemical standard, it plays a part in method validation, calibration, and reference techniques for analytical chemistry. Its reach goes far beyond molecules stuck forever in sample vials; countless projects rely on having access to this building block at consistent purity and supply.
Research & Development
Current R&D themes go where new science bubbles up: medicinal chemistry, synthetic method development, structure–activity relationship mapping, and the drive for greener, lower-waste routes. Researchers continue expanding the substitution pattern, testing new combinations or masking groups based on the 6-chloro core to drive up biological potency or selectivity. Academics publish studies about enzyme-catalyzed functionalization, and industry looks into process intensification, recycling, and solvent recovery for cost and sustainability reasons. Many papers talk about creating combinatorial libraries—huge sets of related compounds—for high-throughput screening in pharmaceutical settings. Investment in analytical protocols, using LC-MS, FTIR, or single-crystal x-ray, ensures confident identification, purity checks, and reproducibility. Collaborative efforts linking computational modeling, synthetic chemistry, and real-world biology squeeze every drop out of 6-chloroindolin-2-one to deliver the next breakthrough target.
Toxicity Research
Every new or reintroduced molecule needs toxicological data to spot risks for both people and the environment. Studies on 6-chloroindolin-2-one show the compound can irritate tissues on contact, warning researchers to use proper PPE and handle even trace quantities with respect. In-vitro and animal studies hint at low to moderate toxicity, with close tracking of the dose and route of exposure. Some early animal tests check for mutagenicity, teratogenicity, and chronic toxicity, but the evidence hasn’t pinpointed acute dangers outside mishandled laboratory use. Environmental breakdown studies place a focus on wastewater treatment, because halogenated organics may persist under certain conditions if not managed carefully. Regulatory agencies and producers keep updating guidance documents to reflect new health hazard data, pushing toward safer chemistries where possible.
Future Prospects
Folks on the research and manufacturing side see a growing need for specialty building blocks with clear, reproducible properties and clean safety data. With its versatility and well-understood chemistry, 6-chloroindolin-2-one will remain a key player in both medicinal innovation and synthetic method development. Projects investigating new cancer, CNS, or inflammation drug candidates continue leaning on the oxindole family for initial leads and SAR exploration, and as the green chemistry movement keeps reshaping processes, scalable, less-hazardous syntheses get more attention from both regulators and buyers. Global supply chain resilience pushes producers to improve documentation and transparency on origin, impurity, and sustainability metrics. Industry-watchers expect more custom derivatives tailored to emerging disease targets, and even researchers in material science keep coming back to these reliable core motifs in the hunt for nimbler organic semiconductors and functional dyes. My time in the lab confirms the demand for purity, processability, and clarity never fades, and every step toward safer, more sustainable use of compounds like 6-chloroindolin-2-one lifts the bar for what modern chemistry can offer.
Peering Into the Structure
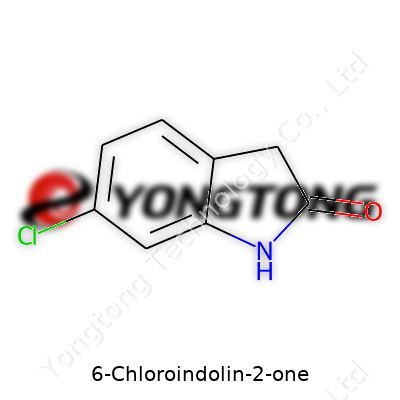
6-Chloroindolin-2-one might sound unfamiliar if you haven’t spent much time with chemistry books, but the way it’s built explains so much about its behavior. Chemists group it under the indolinone family, and for good reason. Its backbone is an indolin-2-one core—a bicyclic structure with a six-membered benzene ring joined with a five-membered nitrogen-containing ring. The twist comes with a chlorine atom sitting right at the sixth spot on the ring.
Let’s look closer. The molecular formula reads C8H6ClNO. Imagine the benzene ring as a sturdy scaffold. At its sixth carbon, a chlorine atom replaces a normal hydrogen. This chlorine isn’t just decoration. It brings a strong electron-withdrawing effect, changing how the molecule reacts with others. The core indolin-2-one block comes from a fusion between benzene and a lactam (a cyclic amide), which plays a role both in pharmaceuticals and organic synthesis.
From Structure to Significance
A lot of important molecules are built off this shape. I remember struggling with similar molecules during organic chemistry—once I saw how small tweaks like adding a chlorine could influence reactivity, the importance became clear. Chlorine alters the electron distribution across the ring, often boosting the substance's biological activity. That’s why pharmaceutical researchers study it for new drug leads. A molecule like this can fit into enzymes, bounce into different receptors, or even change its effect based on this single atom switch.
It’s more than curiosity—it’s practical. The chlorine makes 6-Chloroindolin-2-one a strong starting point for demonstrating how small chemical changes leave big footprints in drug development. This molecular skeleton shows up in antitumor agents and even some nonsteroidal anti-inflammatory drugs. The pharmaceutical industry doesn’t just want one magic bullet; they’re after whole classes of new medicines. 6-Chloroindolin-2-one is a reliable workhorse in those labs, acting as a scaffold for newer, refined molecules.
Challenges and Solutions
Working with chlorine-containing compounds never comes easy. 6-Chloroindolin-2-one can be tricky to make in the lab, and dealing with chlorinated waste calls for thoughtful disposal practices. Safety comes first, especially since chlorinated aromatics can linger in the environment. It helps to follow green chemistry techniques, like choosing less toxic solvents and trying to cut down on excess reagents. Labs investing in cleaner synthesis routes don’t just help the environment—they cut long-term costs and improve working conditions for chemists.
Researchers sometimes face supply chain hiccups when trying to buy specialty molecules like this, especially at high purity. Sourcing from reliable suppliers with strong documentation and transparency makes a difference. I’ve learned in my own work that partnerships with chemical suppliers who prioritize quality control can help projects move forward without the usual resupply headaches.
Beyond the Flask
The chemical structure of 6-Chloroindolin-2-one goes way beyond a classroom exercise. It embodies decades of insight into how slight changes shape both reactivity and medical potential. Researchers can look at this molecule as a gateway to new discoveries, while keeping a close eye on safety and sustainability in their routine. In a world that demands new treatments and greener chemistry, the lessons from molecules like this hit home.
What 6-Chloroindolin-2-one Brings to the Table
Work in chemical research often circles around molecules that can do more than one job. 6-Chloroindolin-2-one stands out for those who try to build new medicines and materials. This compound walks into the lab as a starting point, sparking a string of creative reactions. Its structure—an indolinone with a chlorine at the sixth position—offers building blocks for further innovation.
Pharmaceutical Development
Drug design and synthesis push for molecules that are easy to modify and offer biological activity. 6-Chloroindolin-2-one steps up as a core piece in building a range of indolinone derivatives, many of which show anticancer and anti-inflammatory activity. Pharmaceutical chemists often use it while exploring new inhibitors that target kinases, a big family of enzymes tied to cancer growth. One example is the work on sunitinib, a well-known cancer medication, which draws from an indolinone backbone. With the chloro group sitting on the core, there’s room for fine-tuning how a drug fits into an enzyme or receptor, potentially making medicines more precise and effective.
Agrochemical Contributions
Farms and fields also see the value of indolinone-based molecules. 6-Chloroindolin-2-one gives a handy base for crafting novel fungicides or herbicides. The structure allows chemists to make tweaks that change how a chemical interacts with plant or fungal targets. Field trials often follow lab synthesis, where derivatives get tested for crop protection. Researchers hunt for options that spare helpful organisms but target weeds or diseases, keeping food supplies secure without causing knock-on damage.
Materials Science and Beyond
Polymer chemists sometimes use derivatives of indolinones in efforts to improve material properties. Additions like the chlorine atom can shift how a polymer behaves—things like heat resistance, color stability, or mechanical strength take shape with small changes at the molecular level. This kind of work helps companies design specialty plastics and coatings where performance outstrips older options. It reminds me of the time I worked with a team trying to adjust conductivity in plastic films. Adding small tweaks to the starting molecule, just like with 6-Chloroindolin-2-one, made all the difference in hitting target specs and moving the idea out of the lab.
Supporting Responsible Use
Any chemical with broad applications asks for careful handling and testing. Safety checks and environmental monitoring sit alongside the search for better drugs or materials. Research teams try to reduce toxic byproducts and develop greener synthesis methods. Academic groups and private firms both look for partnerships that speed up progress but keep accountability front and center. Using 6-Chloroindolin-2-one responsibly supports the development of better solutions for health, food, and technology without ignoring the responsibilities to people or the planet.
Looking Ahead
The story of 6-Chloroindolin-2-one reminds us that chemistry shapes everything we touch—medicine, food, gadgets on our desks. Progress depends on smart choices at every step, from molecule design to practical application. This compound sits in the toolkit for anyone hoping to push research and industry forward, grounded by a respect for safety and real-world impact.
Why Purity Matters
Lab work demands more than a label on a chemical bottle. With 6-Chloroindolin-2-one, every decimal in the purity count can shape an experiment, nudge a reaction, or spoil a synthesis. Working in organic synthesis for years, I have seen how an impurity, hardly a speck, can reroute a pathway or fake a clean NMR. In research, certainty has never been cheap, and purity reigns at the top of the order sheet. Pharmaceuticals, agricultural research, or pigment manufacture all set different bars, but a standard hovers above: 98% purity or higher, with 99%+ drawing the crowd seeking top-shelf quality.
Reliable Sources and Analytical Evidence
Chemists running a project for a pharmaceutical intermediate don’t gamble on unknowns. Suppliers of 6-Chloroindolin-2-one like Sigma-Aldrich or TCI commonly provide grades purer than 98%, typically backed by a certificate of analysis (CoA). The CoA reads like a finals scorecard—listing melting point, HPLC, and NMR, plus the small slice of possible byproducts. Purity dips mean lingering starting material, dust from side reactions, or just stubborn solvent. Authenticity shines through clear documentation.
With tighter purity demands, reputable suppliers don’t just estimate. High-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy turn up the proof. On a typical CoA, 99% purity tells you only a trace of other compounds remain. These methods, by reputation and reproducibility, push aside the guesswork of an earlier age.
Price, Supply Chains, and Fair Trade-Offs
Budget shapes choices that textbooks gloss over. A high-purity batch, say at 99.5%, drains the wallet faster than a 97% batch—but the trade-off pays off when you want reliable, surprise-free results. In some stretches, the market runs hot; a sudden burst in demand from drug development or new pigments swings prices up, pulls bulk stocks lower, and forces a choice between cost and need. I’ve paid premiums, wise or not, to trim away post-purchase purification steps and dodge wasted hours.
Local regulations also pull weight. Chemicals landing in U.S. or E.U. labs ship with more details, more scrutiny, and usually higher purity than generic lots coming from unknown brokers on overseas trading platforms. Trust trails behind every batch, and returning to the same trusted supplier often beats the savings dangled by questionable sources.
Setting Realistic Expectations and Solutions
Purification at the supplier level costs money and time. For specialized projects pushing for ultra-high analysis-grade material, buyers should expect longer lead times and a fatter invoice. Planning matters: nobody likes having to re-purify because something “almost pure” led to ambiguity in crucial tests. Where budgets pinch, collaborating with trustworthy local labs for further purification might outclass taking a gamble on cheaper imports.
I’ve learned that keeping extra documentation—spectra, CoAs, batch numbers—not only smooths audits but saves face during collaborations. Researchers, especially in academia or pharma, shouldn’t just ask for purity on paper. Request recent batch analyses, and inquire about the full impurity profile. That extra diligence prevents surprises far better than price-juggling ever could.
Why Storage Routines Shape Safety
Every time I’ve handled powders or compounds in the lab, I notice seasoned researchers pay close attention to storage. They know small oversights create big risks, especially with less common chemicals like 6-Chloroindolin-2-one. This compound, often found in research or pharmaceutical settings, carries hazards that call for clear rules and a few habits that never get skipped. Safety and cost matter equally. Nobody wants an accident, and nobody wants to lose their research budget because of spoiled chemicals.
Understanding the Risks
6-Chloroindolin-2-one looks harmless—a pale powder, often in a tightly sealed bottle. Hidden dangers still exist. No one wants to breathe dust from aromatic organic compounds. Add in the legal requirements for chemical storage and the risk of fire from volatile organics, and the desk drawer stops looking good.
Temperature swings turn a shelf into a hazard. Prolonged exposure to moisture corrupts lots in subtle ways. UV light from the window can put an end to purity, which destroys repeatability in tests. Anyone who’s run into these issues traps in humidity or gets a whiff of an off-odor knows management sets policies for real reasons, not paperwork’s sake.
Pushing for Good Habits
I learned early not to leave containers open—even during short breaks. Even a brief lapse creates an invite for water vapor and contamination. A tightly sealed cap, clearly labeled, and safely away from food or drinks should be automatic for anyone serious about their results.
Nothing replaces a dry, cool storage space. Ordinary room temperature storage might sound reasonable, but stable conditions beat out any guesswork. Classic desiccators deliver on dryness; if desiccants get swapped and lids fit, crystals or powders like 6-Chloroindolin-2-one stay true to form longer. Good refrigeration shrinks chemical aging. I’ve seen the difference: old fridge stored samples work, and those left out just don’t.
Smart Storage Choices
Flammable cabinets make sense when storing organics, especially with compounds that degrade or catch fire under the wrong conditions. These cabinets prevent temperature spikes. I try to keep all small bottles inside the same cabinet, away from acids or strong bases, since one spill can cloud an entire batch.
Clear, updated labeling isn’t just about compliance. Sharpie-written scrawls fade and confuse staff—computer printed cross-checked labels bring clarity and avoid mistakes. Small details, like adding a date, stop expired stock from ending up in someone’s project.
Solutions and Responsible Chemistry
Self-audits matter. Once a quarter, walking through every shelf and fridge clears out out-of-date stocks and reminds everyone which bottles can’t just be left behind after a long day. Training new staff never ends. Every time I’ve seen a near-miss, the lesson gets cemented: strong basics like clear storage plans protect both people and research.
6-Chloroindolin-2-one isn’t a compound anyone treats lightly. Clear labeling, a sealed dry environment, careful refrigeration, and proper disposal ensure safety and scientific integrity. Habits that begin with one chemical end up protecting a whole team, long after a single study ends.
Why Numbers Matter in Chemistry
Every substance in a laboratory carries a unique identifier. For 6-Chloroindolin-2-one, scientists use the CAS number 15335-46-9. This number isn’t just a label. It acts like a passport for the compound—one that allows researchers, regulatory agencies, and chemical suppliers to know exactly what’s in the vial, no confusion or mistranslation. Assigning a clear number to a compound cuts through the fog of language barriers and synonyms that cloud international research.
My Take from Working in a Research Lab
Years in academic labs taught me the frustration that comes from mislabeling or cross-referencing chemicals. Digging through product catalogs, I quickly found that names like “6-Chloroindolin-2-one” sometimes get repeated with slight tweaks: “2(1H)-Indolinone, 6-chloro-”, “6-Chloro-2-indolone”, and so on. It’s exactly this issue the CAS system solves well. With the number 15335-46-9, it’s impossible to confuse the identity of the compound, whether ordering for an experiment or sifting through journal databases.
The Importance Reaches Beyond Research
The story gets bigger outside the bench. Chemical safety depends on precise identification. Regulatory agencies, both local and international, refer to CAS numbers in their chemical inventories and restricted-substance lists. If you work in quality control, toxicology, or procurement, being off by a digit in that CAS registry can mean major headaches—or worse, compliance violations. Public safety depends on tight systems for tracking chemicals, and the simple act of confirming the CAS helps prevent errors from snowballing.
The Facts Behind the Registry
The Chemical Abstracts Service (CAS), operating under the American Chemical Society, assigns these numbers. Think of the CAS Registry as the world's biggest Rolodex for chemicals—over 200 million entries. Each number holds data on structure, physical properties, supplier links, and hazards. If you’re buying, storing, or handling 6-Chloroindolin-2-one, 15335-46-9 links you to all the right technical details and safety data sheets.
Common Pitfalls and How to Solve Them
Mistakes with CAS numbers often start from copying numbers from old paperwork or using outdated sources. One wrong number, and a scientist could be ordering a different indolinone entirely, risking contamination in research or problems in manufacturing batches. Verifying CAS numbers each time with reliable sources—such as the official CAS database or well-known chemical suppliers—remains the sure way to stay out of trouble.
Digital tools now make this job easier. Laboratory information management systems and chemical inventory apps link every chemical to its CAS entry, helping labs and companies create audit trails. Integrating these tools shrinks the human error margin and boosts accountability all the way up the supply chain.
What Can Help in the Long Run
Stronger training for researchers and students on proper chemical labeling methods helps from the earliest days in the lab. Encouraging teams to double-check CAS numbers on paperwork and digital files makes for better habits. If you spot a mismatch, call it out; in lab settings, vigilance on chemical identity shows commitment both to safety and quality science.
CAS numbers—such as 15335-46-9 for 6-Chloroindolin-2-one—remain crucial to protecting people, precision, and progress in chemistry.