5-Fluoroindolin-2-one: A Comprehensive Commentary
Historical Development
Chemists have shown a steady fascination with indolinones since the early days of medicinal chemistry. Researchers began tinkering with the indolinone core after World War II, seeking new pharmacological agents. The introduction of halogens, especially fluorine, opened a new chapter for this family of compounds. The specific appearance of 5-Fluoroindolin-2-one in literature mirrors the growing confidence of synthetic chemists by the 1970s, who leaned on halogenated motifs for tweaking biological activity and dialing up molecule resilience. These experiments reflect the constant struggle to outpace resistance in antimicrobial and anticancer therapies. Academic theses and pharmaceutical patents, particularly in the last two decades, highlight this molecule for its potential to steer the next generation of bioactive substances.
Product Overview
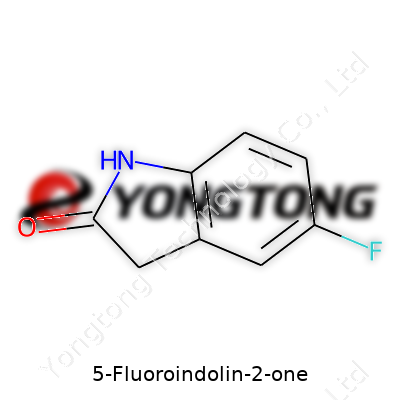
5-Fluoroindolin-2-one stands as a member of the indolinone family sporting a fluorine atom at the 5-position — a simple twist in structure, but with outsized impact on its behavior. Researchers value it as both a tool for discovery work and a precursor for creating more complex structures. Fluorine, a notoriously small yet electronegative atom, tunes binding affinity with biological targets and shifts a molecule’s fate inside the body. This makes 5-Fluoroindolin-2-one a common fixture in chemical catalogues and on the benches of labs testing new therapies.
Physical & Chemical Properties
Experience in the lab reveals some straightforward truths about 5-Fluoroindolin-2-one. It crystallizes into an off-white powder, sometimes taking on faint yellow hues if stored without care. Weighing in at 151.13 g/mol, its modest size suits it for various reaction schemes. Its melting point hovers around 180 °C; heating above this transforms the solid into a dark, viscous oil. Solubility favors organic solvents over water, making DMSO, DMF, and chloroform the first stops for most dissolve-and-stir protocols. As for stability, the compound holds up well under common storage conditions, but exposure to open air or moisture for extended periods leads to slow degradation, a factor chemists quickly learn to manage to preserve material integrity.
Technical Specifications & Labeling
Bottles carrying 5-Fluoroindolin-2-one in reputable labs sport clear labeling in line with global safety guidelines: purity percentage, batch number, storage recommendations, and expiry dates. Quality material logs a purity of at least 98%, confirmed with HPLC or NMR analysis. Safety symbols point to both irritant and slightly toxic properties, steering users to don gloves and goggles, a routine as familiar as tying one’s shoes before work. Product codes often reference supplier-specific catalogues, so researchers cross-check CAS numbers (51633-35-5) to avoid handling near-duplicates or misordered compounds.
Preparation Method
Organic chemists know there’s rarely a single, foolproof route to a target molecule. Synthesis of 5-Fluoroindolin-2-one typically starts from 5-fluoroindole or its derivatives, using well-worn oxidation and cyclization tactics. A common path involves phenylhydrazine chemistry, with nitrosation followed by cyclization under mild acid or base. Modifying the process (using different oxidants or catalysts) sometimes bumps up yields or shortens reaction times, reflecting chemists’ drive to snag higher purity and efficiency. Multi-gram quantities require a good eye for temperature control and plenty of patience during the purification step, usually a repeated dance with silica chromatography.
Chemical Reactions & Modifications
The true promise of 5-Fluoroindolin-2-one becomes clear once it enters the realm of chemical modification. The carbonyl at C-2 serves as a hub for nucleophilic addition, so savvy chemists generate libraries of analogs by plugging in diverse substituents. The molecule offers a playground for Grignard reactions, reductions to amines, or coupling with sulfonyl and acyl groups. Fluorine at the 5-position tends to resist displacement, but its electronic impact ripples through the ring, subtly nudging reactivity at other spots — a nuance uncovered one reaction by one. This has a direct bearing on medicinal chemistry where every electron counts in the endless contest to improve bioavailability or sneak past metabolic defenses.
Synonyms & Product Names
5-Fluoroindolin-2-one carries a basket of synonyms thanks to decades of global research. Names crop up in international regulatory filings and academic articles: 5-Fluoro-2-indolone, 5-Fluoro-1,3-dihydro-2H-indol-2-one, and sometimes CAS shorthand. Catalogues and procurement platforms list it under these labels, and recognizing all these aliases can save hours of fruitless searching or avoid costly mis-orders. The compound’s common names reflect both structural logic and the cautious creativity of chemists who balance tradition with the need for clarity.
Safety & Operational Standards
Lab safety for 5-Fluoroindolin-2-one tracks with general practice for small organic molecules carrying halogen and ketone groups. Gloves and safety glasses form the basic armor, but longer handling or scale-up batches call for fume hoods to banish dust and vapor. Researchers heed warnings about potential skin, eye, and respiratory irritation. In my own experience, splashing a few milligrams prompts an instant trip to the eye-wash, no questions asked. Good inventory habits and double-checking MSDS sheets stop accidents before they start. Waste handling sends spent solutions to organic waste bins; drain disposal isn’t just frowned on, it risks serious regulatory trouble.
Application Area
5-Fluoroindolin-2-one’s true value emerges in the dizzying pace of drug development. Medicinal chemists graft its core into candidate molecules for anti-cancer, anti-infective, and anti-inflammatory research. Tools for kinase inhibition screening feature it as a starting point, since fluorinated indolinones routinely turn up among the most promising hits in cancer trials. Outside pharmaceuticals, some researchers branch into agrochemicals and dye chemistry, though its high-value status keeps it mostly in the hands of biotech firms and research organizations. Academic papers and patent filings chart an ever-expanding field of possibilities tied to subtle ring modifications.
Research & Development
Current R&D efforts treat 5-Fluoroindolin-2-one as a core building block. Scientists focus on optimizing its biology activity, using SAR (structure-activity relationship) studies to spot winning combinations of fluorine placements and ring substituents. The molecule’s manageable reactivity allows for late-stage diversification, a handy trait as discovery teams try out rapid analog generation. Platforms such as AI-driven drug design chew through clouds of virtual indolinone derivatives and spit the most promising ones back to the bench. This molecule often serves as a benchmark for what a “drug-like” fluorinated heterocycle looks like in absorption, distribution, metabolism, and excretion studies. In fields like chemical biology, researchers deploy it as a probe for protein interactions, sometimes attaching fluorescent tags or radioisotopes to follow its journey through cells.
Toxicity Research
Toxicologists dig deep into 5-Fluoroindolin-2-one’s effects using cell cultures, animal models, and in silico predictions. The addition of fluorine reduces metabolic breakdown in the liver, sometimes extending both positive effects and risks. LD50 data sets show relatively low acute toxicity in rodents, but long-term studies remain sparse. This hole in the data matters, especially with agencies tightening scrutiny on new chemical entities. Off-target effects—such as binding stray proteins or triggering immune responses—are a concern flagged in early preclinical reviews. Repeated exposure sometimes produces mild liver changes and subtle neurological symptoms in test animals. Toxicity studies guide the design of safer derivatives and steer efforts to protect lab workers, reinforcing the mantra that there are no harmless chemicals, only careful chemists.
Future Prospects
The role 5-Fluoroindolin-2-one occupies in chemistry looks set to expand. Advances in synthetic methods are lowering the barriers to analog creation, making it easier for early-career scientists or smaller labs to enter the field. Demand for fine-tuned kinase inhibitors virtually ensures this molecule or close relatives will fuel the next round of patent races in oncology and inflammatory disease. Fluctuating regulatory environments will push for more in-depth toxicological profiles, possibly nudging suppliers to invest in greener synthesis and more transparent sourcing. The rise of computational chemistry puts molecules like 5-Fluoroindolin-2-one through digital stress-testing before actual synthesis, trimming research timelines and directing human effort. As fields from neuroscience to plant science hunt for new leads, the simple, tough framework of fluorinated indolinones stands ready—waiting for the next breakthrough insight or serendipitous discovery.
The Building Block in Pharmaceutical Research
5-Fluoroindolin-2-one tends to pop up on the radar of anyone working at the edge of medical research or pharmaceutical chemistry. Its structure looks a bit unassuming, yet the chemical world often puts a strong emphasis on what such small, seemingly odd compounds can achieve. Over the years, 5-Fluoroindolin-2-one earned its stripes as a reliable building block, especially among teams working on anti-cancer agents and new antibiotics.
I remember my own weeks spent in a lab hoping to breathe new life into an old drug molecule. My supervisor handed me a sample of something called 5-Fluoroindolin-2-one and told me this was the starting point. We worked with it to craft analogues, tweaking bonds and seeing what sort of power lay hidden. The funny part was watching the bioassay results. Sometimes the switch of a fluorine atom, like in this compound, tipped the scales between a weak inhibitor and a totally new class of potent therapeutics.
Why Chemists Value 5-Fluoroindolin-2-one
Medicinal chemists often reach for this molecule because it brings options to the table. Indolinones, in general, appear often in kinase inhibitors, and those inhibitors have shaped the treatment patterns for some cancers. Introducing a fluorine atom onto the core structure usually bumps up the compound’s metabolic stability. This leads to longer lasting effects inside the body, which translates to better days for patients slated to take these medications.
The fluorine atom also flips some switches in how molecules interact with proteins. Researchers build “libraries” of slightly different molecules using compounds like 5-Fluoroindolin-2-one. They search for ones that slow down or stop dangerous enzyme activity, such as mutated kinases found in tumors. I recall a meeting where one of our group leaders spelled out the latest findings: Fluorinated indolinone derivatives sometimes blow past unmodified versions, binding more tightly and sticking around longer in mouse models. This can quicken the march toward clinical trials.
Emerging Uses and Real-World Hurdles
Beyond cancer meds, antimicrobial resistance has pushed teams to test out fresh chemical scaffolds. 5-Fluoroindolin-2-one formed the backbone of several new compounds that take a shot at resistant Gram-negative bacteria. In a few published studies, these molecules performed well in petri dishes and early animal studies, which points to a much-needed avenue for next-generation antibiotics.
Still, translating these discoveries into pills or injections that help real people never feels simple. The biggest challenge remains the step from lab bench to bedside. Toxicity, manufacturing scale-up, and costs all stand in the way. More basic research, alongside smart investment from public health groups, could bring some of these compounds closer to pharmacies. Building deeper links between academia and small biotech firms can also speed things up. Sometimes a good idea sits stuck just because the right people have never been in the same room together.
The Importance of Basic Chemical Research
Too often, flashy results from clinical studies overshadow the slog of foundational work. Compounds like 5-Fluoroindolin-2-one illustrate why slow, thoughtful investigation is so vital. They are the scaffolding of future breakthroughs. As resistance grows and diseases shift, stockpiling a toolkit of well-understood chemical building blocks gives researchers a leg up. Whether for fighting tough tumors or protecting against superbugs, seeing the value in these quiet molecules helps pave the way for treatments tomorrow’s patients will need.
Looking Under the Hood of a Modern Molecular Player
Digging into molecules like 5-Fluoroindolin-2-one feels a lot like cracking open a time capsule for chemists. This one’s got a simple backbone at first glance, but with a twist: a burst of fluorine stitched right where it matters. Lay it out, and here’s what you see—a bicyclic scaffold that brings together an indoline ring, a fluorine atom, and a ketone group where the action happens.
Folks who’ve spent years peering under microscopes or scribbling reaction mechanisms will tell you the secret sauce in drug ingredients often lies in these tweaks. Here, chemists start with indolin-2-one—a molecule with an indole nucleus, just tweaked so it’s a saturated indoline fused to a ketone at the 2-position. That’s a mouthful, but picture this: a six-membered benzene ring fused to a five-membered nitrogen ring. At spot five on the benzene ring, slide in a fluorine atom. This small switch changes everything.
Why a Fluorine Atom Packs a Punch
There’s nothing random about tossing a fluorine atom into a molecule. In the lab, chemists know that swapping a hydrogen for fluorine can crank up a compound’s power. The fluorine atom is tiny but mighty. It yanks electrons harder than just about anything else in the periodic table. That means you get real changes—how the molecule behaves in water, how tough it is to break down, even how it latches onto enzymes inside cells. Pharmaceuticals use moves like this to make drugs last longer in the body or slip through cell membranes with less fuss. We’ve seen fluorinated indolinones pop up in drug discovery, cancer research, and enzyme inhibition. With first-hand experience, navigating journals and research labs, I’ve watched teams transform dead-end leads into real medicines using tweaks like this.
Drawing It Out: What Does 5-Fluoroindolin-2-one Actually Look Like?
For chemists, structure means everything. 5-Fluoroindolin-2-one has this as its backbone: a six-membered ring fused with a five-membered ring with a nitrogen stuck at the first position. The ketone sits at the second carbon—straightforward so far. The fifth carbon on the benzene ring claims a single atom of fluorine, going in for what could have been a hydrogen. This shift creates a slightly uneven ring, giving the whole molecule a dipole and changing its fate in reactions.
Importance for Research and Medicine
Structures like this one have rippled through the pharmaceutical world. By introducing fluorination, scientists have been able to discover new ways these molecules block proteins involved in diseases like cancer. In drug design, every corner on the ring, every atom you swap out, makes a difference in how the body reacts—think less toxicity, better targeting, stronger impact. The evidence stacks up across clinical studies, patent filings, new pharmaceutical compounds. I’ve seen investment flow in for fluorinated building blocks like this because success comes down to precision. These aren’t just building blocks; they’re launching pads for the next generation of medicines.
Addressing the Synthetic Challenge
Making fluorinated indolinones isn’t a kitchen-table task. The right reagents and tight temperature control make a huge difference between a workable yield and a mess. Chemists now have greener, cleaner approaches to fluorination, shrinking waste and toxic byproducts. With education, updated lab protocols, and better sharing of reaction schemes, more labs can safely tinker with structures like 5-Fluoroindolin-2-one.
Moving Forward: Solutions That Work
Educational outreach needs to reach students early. Molecules like this aren’t just abstract concepts—they’re keys for fighting real-world problems. Funding, lab safety, and public awareness matter more than ever. With transparent science and collaborative spirit, molecular discoveries like 5-Fluoroindolin-2-one can be put to work, saving lives and pushing boundaries. It’s not magic; it’s smart, evidence-driven chemistry making a mark on the world.
The Real World of Chemical Storage
Laboratories and warehouses see a parade of chemicals every year. Handling agents like 5-Fluoroindolin-2-one takes more than tossing bottles into a cabinet. It calls for good judgment, a nod to safety, and respect for the quirks of each compound. If you look at what 5-Fluoroindolin-2-one brings to the table, you see a molecule often used for research into pharmaceuticals and organic synthesis. Because of its uses, folks want to keep it as pure and unaltered as possible.
Temperature: A Good Place to Start
Let’s skip the guesswork—don’t leave fine chemicals to fight with humidity or temperature swings. 5-Fluoroindolin-2-one stays solid at room temperature, but even then, room temperature means slightly different things in different corners of the globe. From my own experience with similar indole derivatives, cooler conditions keep them lasting longer. Aim for a place below 25°C if you want peace of mind. Most labs rely on a dry, dark cupboard or a refrigerator set around 2–8°C for good reason: cold slows down harmful reactions.
Light and Moisture: Quiet Enemies
Sunlight doesn’t do chemicals many favors. I’ve opened dusty shelves and seen faded or degraded labels, sometimes even cracked containers. 5-Fluoroindolin-2-one should avoid light as much as possible. A brown bottle works well, but never underestimate the impact of a closed cabinet. High humidity can lead to clumping, hydrolysis, or slow reactions with the air itself. Keep caps tight and consider silica gel packets nearby for extra peace of mind.
Stability and Shelf Life: Lessons from Experience
Stories float around labs of misplaced compounds losing color or changing odor. 5-Fluoroindolin-2-one isn’t explosive or especially volatile, but no chemical enjoys sitting open or in a warm, muggy storeroom. Unopened, stored well, most solid organic compounds like this can go months or even a couple of years without losing quality. Opened vials face extra risks— exposure to air can sometimes kickstart slow changes, even if you don’t see them right away. Labeling every container with the date, source, and initial condition feels like overkill, yet it pays off. I’ve seen researchers save money and avoid headaches this way.
Safe Storage Practices: Beyond the Basics
A tidy storage system keeps mishaps down. 5-Fluoroindolin-2-one doesn’t need a dedicated hazardous cabinet, but curious mixes with acids, strong bases, or oxidizers can set off trouble. Keep similar organics on a shared shelf and trust in sealed containers. If kids or untrained folks share the building, add locks or at least a sign. If a spill happens—a dust mask, gloves, and damp cloths deal with it before dust gets kicked up. Clients and suppliers I’ve worked with all state the same: preparation trumps cleanup.
Takeaways—and a Nod to Regulations
Manufacturers and researchers fall under regional safety agencies, and for good cause. Reading safety data sheets before each batch gets stashed away gives you specific storage hints and warnings. Regulations look dry on paper, but the real cost of ignoring them—lost samples, failed syntheses, or more serious incidents—shows up sooner or later.
Building the Right Habits
5-Fluoroindolin-2-one isn’t difficult once you know its limits: cold, dark, dry, and labeled. Mistakes usually come from complacency, not malice. Those habits taught by a sharp lab supervisor or brought from stories of lost batches sometimes matter even more than any rulebook. Treat every new shipment with the respect it deserves, and most troubles will never show up.
Why Chemical Safety Deserves Attention
In labs and production floors, chemicals like 5-Fluoroindolin-2-one quietly play a role in everything from research to manufacturing. Most people won’t recognize its name, but that doesn’t mean its risks should go unnoticed. Safety with chemicals doesn’t just happen. It’s built day by day, with knowledge leading the charge.
What We Know About Toxicity
5-Fluoroindolin-2-one belongs to a class of compounds researchers often investigate for pharmaceuticals or materials science. There's a problem, though—a lot of specialty chemicals lack detailed safety profiles, and this one is no exception. Without robust animal studies, long-term exposure data, or standardized workplace hazard sheets, workers and lab techs stand exposed. In my experience, handling poorly understood compounds teaches you one core idea: never let uncertainty stand in for proof of safety.
Evidence from related indolinone compounds suggests a mixed bag—some variants are relatively innocuous, some irritate skin or lungs, and others could trigger reactions in the body’s cells. A fluorine atom in the molecule tends to raise eyebrows. Fluorinated chemicals sometimes hang around in the environment and inside living bodies longer than their non-fluorinated cousins, raising flags for potential persistence and bioaccumulation. Toxic effects often depend on how people work with them—whether it’s powders in the air or residues on hands.
Where Risks Show Up
A solvent splash, a bit of dust, or a spill almost always seems more trivial than it turns out. I’ve seen colleagues brush off minor exposure without pausing to read a label or check a safety sheet. Over time, routine handling without respect for hazards invites trouble. Irritation is the first sign with many chemicals, and respiratory effects or skin problems can follow. Since many chemical exposures don’t show their worst side right away, the risk can lurk unseen, manifesting later down the line as dermatitis or deeper tissue impacts.
Making matters tricky, some chemicals stay in the air after a spill. Fine powders, for example, creep far from where they started. If there’s one lesson from long days in the lab, it’s that hurrying to finish a task usually leads to mistakes, and chemical hygiene goes out the window in a rush. The cost lands on health and safety.
How to Approach Safer Use
No one should feel forced to play scientist with their safety. Basic protections go a long way—gloves, eye shields, well-ventilated workspaces. I always urge new staff to ask questions, especially about substances without published toxicity data. Responsibility means checking every time, not just assuming yesterday’s chemical is harmless today. Libraries and chemical suppliers often share bulletins or reports, and using these resources can bring hidden dangers to light. A routine check through databases like PubChem or the European Chemicals Agency also helps, especially when official safety data sheets are missing.
In every workplace, building a culture of speaking up about uncertainty may prevent injuries or slow-developing illnesses. Since a single exposure can have long-term effects, we owe it to each other to set a high bar for precaution—especially with little-known chemicals. If in doubt, treat the unknown as hazardous. In my view, the safest labs and production lines are the ones where curiosity extends to safety, not just the science itself.
Understanding Purity Numbers
In chemical synthesis and pharmaceutical research, purity makes or breaks the outcome. Researchers often look for 5-Fluoroindolin-2-one with a stated purity of at least 98%. This kind of specification doesn't come from arbitrary benchmarks—it reflects what professionals find reliable enough for advanced work. Laboratories trust such benchmarks because even small traces of impurity can throw off results or introduce unpredictable side effects during compound development.
Why 98% Purity Is Not Just a Number
A 98% purity level spells confidence for anyone working downstream, whether fine-tuning a new medicinal molecule or piecing together data for a regulatory filing. I remember handling compounds during my undergraduate research years, and a batch falling below the claimed purity kept causing inconsistent results. Chasing down the source of the inconsistencies wasted days, underlining why published numbers matter.
When scientists receive 5-Fluoroindolin-2-one that meets this standard, risk tapers off. The science gets easier, and findings hold up during review and replication. Regulatory agencies prefer this attention to detail; they want clear documentation of every compound’s chain of custody and test result. FDA reviewers, for example, need a compound’s purity report before even considering moving a molecule forward in the development pipeline.
How Suppliers Prove Their Numbers
Suppliers don’t just take a guess at the purity—they rely on analytical methods like HPLC (High Performance Liquid Chromatography), NMR (Nuclear Magnetic Resonance) or Mass Spectrometry to back up their claims. These reports usually ride along in the batch’s paperwork, so buyers can check and verify. If these records aren’t available, it signals a supplier who might be cutting corners.
Researchers rely on receiving this data every time a new flask lands on their bench. With clear reporting, labs can flag discrepancies early. I once worked with a team who requested these analysis reports alongside every shipment, making it non-negotiable for supplier contracts—this practice ended surprises and kept projects on schedule.
Risks Connected to Lower-Purity Materials
Mistakes often start small. Unidentified byproducts in a lower-purity batch can act as reaction poisons or sneak into a drug candidate, raising toxicology risks. Synthesis reactions sometimes break down, bringing delays or lost money. The headache grows during scale-up, when an impurity that didn’t matter on the gram scale can trigger failures on the kilogram scale. Less experienced teams who skip purity checks sometimes find themselves repeating the basics, wasting resources that could have gone into new discoveries.
What Buyers Can Do
Trusting a supplier’s word is never enough. Checking up on certificates, asking for independent sample analyses, and building relationships with suppliers who always deliver what they promise should be routine. Academics and industry veterans often share stories—good and bad—about suppliers in online chemistry communities. Those reviews help separate outfits who stand by their numbers from those who don’t.
In my experience, asking questions up front not only ensures the quality of each order—it gives researchers a stake in shaping the standards the industry follows. Setting a strict expectation for purity, collecting thorough documentation, and responding quickly to any hint of trouble turns a routine order into a foundation for serious, trustworthy science.