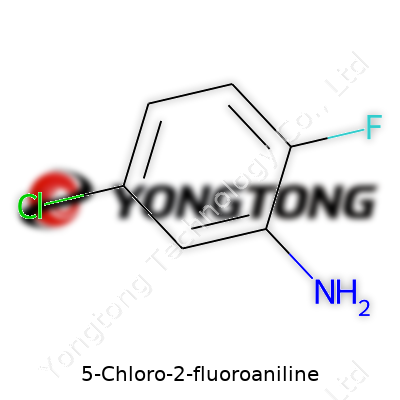
5-Chloro-2-fluoroaniline: An In-Depth Look
Historical Development
Chemists didn’t discover 5-chloro-2-fluoroaniline out of thin air—its journey began decades ago as part of the global quest for new intermediates in organic synthesis. Advances in aromatic chemistry during the twentieth century propelled researchers to modify aniline rings, hoping to find compounds that could go beyond dyes and resins into fields like pharmaceuticals and crop protection. Fluorination and chlorination, two influential chemical tweaks, became commonplace in labs seeking to create molecules with more diverse reactivity and enhanced stability. The intersection of these trends brought 5-chloro-2-fluoroaniline into focus. Its presence in countless patents and technical bulletins reflects practical utility born from persistent laboratory effort, sharpening the toolset for organic synthesis and downstream technology.
Product Overview
5-Chloro-2-fluoroaniline stands out as a halogenated aromatic amine prized by chemists and industrial operators. The molecule holds a benzene core with chlorine perched at the 5-position and fluorine at the 2-position, plus an amine group. It serves as an intermediate compound—essential for building pharmaceuticals, certain pesticides, and specialty chemicals. The careful combination of the two halogens tips the balance of electron density around the ring, shaping reactivity and influencing safety profiles, something no simple aniline derivative could provide.
Physical & Chemical Properties
This compound usually appears as a pale yellow to light brown crystalline solid, sometimes tending toward waxy textures in less-pure batches. It carries a faint, sharp odor typical of chlorinated anilines. Melting temperatures run in the range of 35-39°C, while boiling hovers near 240°C. Its solubility in common organic solvents like ether or acetone is moderate, but it rarely dissolves much in water due to hydrophobic tendencies mixed with limited hydrogen bonding. Under standard conditions, it resists rapid degradation but reacts in the presence of strong acids, bases, or oxidizing agents. The combination of chlorine and fluorine changes the electron-withdrawing nature of the ring compared to simple anilines, allowing for different kinds of downstream conversions.
Technical Specifications & Labeling
Specifications matter a great deal in any operation handling raw chemical feedstocks. Purity levels for 5-chloro-2-fluoroaniline usually sit at or above 98%, with maximum allowed impurities listed on most certificates of analysis. Commercial grades arrive in sealed steel drums or high-density polyethylene containers under nitrogen purge, protecting against oxidation and moisture ingress. Labels carry hazard pictograms for acute toxicity, as well as recommendations for using proper personal protective equipment. For shipping, compliance with standards like GHS and relevant codes for transport by sea or air remains a must. Certificate of Analysis documents generally include melting point, assay, impurity thresholds, and batch tracking information.
Preparation Method
Manufacturers take several routes to synthesize this compound, but direct halogen substitution on pre-functionalized anilines proves efficient. A typical process involves nitrating a fluorinated benzene, followed by chlorination at the desired position, then reducing the nitro group to an amine. Some workflows start with o-fluoroaniline, using selective chlorination to introduce chlorine at the right spot. Careful temperature control, solvent selection, and reagent purity ensure minimal formation of isomeric byproducts. The resulting mixture undergoes fractional distillation or crystallization for further purification. Regulatory pressure has also led companies to reduce use of harsh reagents, adopt catalytic hydrogenation where possible, and capture effluent streams for solvent reclamation or safe chemical disposal.
Chemical Reactions & Modifications
5-Chloro-2-fluoroaniline’s chemical backbone opens a suite of possibilities for further chemical transformation. The amine group often serves as a handle for coupling reactions, especially in the formation of amides or ureas for drug candidates. The ring can take on new substituents using electrophilic aromatic substitution, although the combined effects of the chloro and fluoro groups make orienting such reactions a precise challenge. In medicinal chemistry, Suzuki and Buchwald–Hartwig couplings use the bromo or iodo analogs, but starting from 5-chloro-2-fluoroaniline remains cost-effective for certain series. Some synthetic approaches attach bulkier groups to the free amine using standard protecting-group strategies. Deactivation by fluorine also slows down unwanted side reactions, which can be helpful for careful stepwise synthesis.
Synonyms & Product Names
Like many specialty chemicals, this material appears under a range of alternative names, often reflecting subtle differences in supplier catalogues. Popular synonyms include 2-fluoro-5-chloroaniline, 5-chloro-o-fluoroaniline, and by its IUPAC name, 5-chloro-2-fluorobenzenamine. CAS registry numbers offer a universal key for tracking lots and sourcing batches globally. Large distributors, including Sigma-Aldrich and Tokyo Chemical Industry, may market it under different grades, tailored to research, pilot plant, or bulk manufacturing clients. Search engines, chemical suppliers, and regulatory authorities rely heavily on these synonyms and product codes for smooth commerce and oversight.
Safety & Operational Standards
5-Chloro-2-fluoroaniline cannot be handled like table salt. Its acute toxicity calls for strict safety protocols throughout storage, handling, and process integration. Prolonged or repeated skin exposure can cause irritation and sensitization, while inhaling dust or aerosolized forms may threaten respiratory health. Operators wear chemical-resistant gloves, goggles, and sometimes full face shields. Proper ventilation directly impacts occupational health, often requiring local exhaust and full enclosure during bulk transfer. In case of contact, immediate washing with soap and water joins protocols for eyewash and emergency showers nearby. Emergency responders need to know the compound’s key hazards, with MSDS and GHS labeling visible at all times. Waste streams carrying this chemical go to certified disposal facilities under RCRA or related regulatory controls.
Application Area
Demand for 5-chloro-2-fluoroaniline tracks closely to high-value segments in crop protection chemicals, specialty dyes, and pharmaceutical intermediates. Many herbicides use halogenated anilines as anchor points for bioactive molecules tailored to new plant pathogens or shifting crop landscapes. Some modern painkillers and anti-inflammatory drugs draw on this intermediate to reach desirable metabolic stability and selectivity. Polymer researchers, intrigued by how subtle chemical modifications tweak performance, choose halogen-anilines for specialty coatings and plasticizers. Industrial research teams often treat 5-chloro-2-fluoroaniline as a workhorse intermediate, passing through quickly on the path to larger, more valuable molecules.
Research & Development
Lab notebooks and patent databases show hundreds of studies on this molecule’s derivatives and reactivity. Academic partnerships with industry continue to push boundaries, trying to unlock greater selectivity in downstream reactions or tap new space in fluorinated pharmaceuticals. Green chemistry plays a growing role, with new catalyst systems and solvent combinations aiming to reduce environmental load and improve isolation yields. Automated screening of analog libraries often starts with halogenated anilines, giving researchers a head start in evaluating biological activity across a wide swath of targets. Analytical chemists use advanced chromatography and spectroscopy for tracing impurities and confirming structural assignments, enabling a tight feedback loop between bench and plant floor.
Toxicity Research
No discussion of aromatic amines lacks caveats about toxicity. Animal studies and in vitro screens show moderate to high acute toxicity for 5-chloro-2-fluoroaniline, with evidence that metabolism can form reactive intermediates. Genotoxicity remains a research concern, given the pattern observed with related compounds, necessitating care in both workplace exposure and environmental release. Ongoing investigation seeks to clarify chronic effects, breakdown products in wastewater, and impacts on aquatic ecosystems. Industrial hygiene measures have kept workplace exposures generally low, but continual improvements in containment, monitoring, and spill response reduce risk further. Regulatory bodies update guidance frequently, reflecting the evolving risk landscape as new toxicology data comes in.
Future Prospects
As more pharmaceutical and agrochemical pipelines lean on innovative halogenated building blocks, 5-chloro-2-fluoroaniline looks set for sustained relevance. The continued refinement of synthesis routes—favoring greener solvents, greater atom economy, and easier waste reclamation—can open new markets where regulatory regimes tighten on hazardous intermediates. Researchers will likely harness machine learning and automated reaction platforms to personalize synthetic pathways, boosting efficiency and agility. For end-users, supply chain transparency—from raw material provenance to container labeling—will matter more than ever in light of global scrutiny on chemical safety. The next decade will bring not only new molecules born from this intermediate, but also smarter, safer, and more sustainable ways of making and moving them through the complex world of today’s chemical industry.
Getting to Know 5-Chloro-2-fluoroaniline
5-Chloro-2-fluoroaniline doesn't attract much attention outside chemistry circles, but its impact goes farther than a simple line drawing on paper. Picture a benzene ring, known for its stable hexagonal shape. On this ring, a chlorine atom attaches at position five, a fluorine angle in at position two, and an amino group anchors itself at position one. This setup gives us C6H5ClFN. It sounds straightforward, but each atom affects how this molecule behaves — who it bonds with, what it resists, and what it might become when put through a reaction.
Why 5-Chloro-2-fluoroaniline Matters
I’ve watched researchers use compounds like this one as building blocks for dyes, pharmaceuticals, or even pesticides. Synthetic chemists often face these small molecules before anything hits a medicine shelf. The chlorine at position five doesn't just tweak the electrical charge around the molecule; it changes how easily other groups can squeeze in for further reactions. With fluorine, things get trickier. A fluorine atom likes to snatch electrons and stick tight. By stacking both halogens on the same ring, chemists get something sturdy, less likely to break down in harsh conditions, maybe more soluble in certain solvents than you’d expect from just standard aniline.
Concerns Raised By Halogenated Aromatics
Both chlorine and fluorine bring their own baggage. Halogenated aromatics don't break down as quickly in nature compared to their non-halogenated cousins. That means any spill or byproduct can hang around for a while, lurking in water or soil. It reminds me of times I’ve seen industrial parks run into trouble with chlorinated waste leaching from storage drums. Once out in the world, getting rid of them isn’t always cheap or easy. The use of such compounds gets scrutinized for health effects, too. Inhaling or handling things like 5-chloro-2-fluoroaniline without gear can cause real harm, so safe handling isn't just a suggestion — it's standard practice supported by years of accident reports and lab guidelines.
Holding Science Accountable
Any company or lab using 5-chloro-2-fluoroaniline should track every gram. Regulations exist for a reason. Tracking use helps stop waste from winding up in water tables or food chains. I remember one chemist who took this discipline to heart, labeling every batch and documenting disposal with a vigilance that made the supervisor proud — no shortcuts, no hazardous surprises for the environment. That approach limits accidental exposures and keeps people out of the hospital.
Building a Safer Future in Chemical Design
It’s possible to design new molecules that perform the same jobs as 5-chloro-2-fluoroaniline but break down more cleanly once discarded. Green chemistry isn’t just a buzzword anymore. Teams worldwide actively search for options that use fewer toxic reagents but deliver the same punch, whether it’s in a drug or a dye. That takes both knowledge and willingness to invest in better outcomes, not just quicker profits. Each time someone selects a safer substitute, or catches a spill before it grows, we take a step toward a world where useful doesn’t mean dangerous by default.
Finding 5-Chloro-2-fluoroaniline in Daily Products
Step into most research labs or chemical plants and you’ll find shelves lined with specialty chemicals, including 5-Chloro-2-fluoroaniline. I encountered this compound during an internship at a mid-sized agrochemical business. The project team relied on its structure to create molecules designed to manage pests in staple crops. The reason: the 5-chloro and 2-fluoro positions on its aromatic ring can unlock different biological effects. This is not just a theoretical benefit—testing showed certain derivatives could outperform older pest management formulas. Farmers end up harvesting healthier crops with fewer chemical applications, which keeps food safer and lowers costs.
Key Ingredient for Custom Molecules
Modern drug development uses building blocks like 5-Chloro-2-fluoroaniline. Its reactive amine group combined with the chlorine and fluorine atoms make it a favorite for medicinal chemists. Whenever teams at pharmaceutical companies work on new candidates for cancer or respiratory disease drugs, they want flexibility for rapid fine-tuning. I watched colleagues use this compound as a stepping stone, adding different side chains or ring systems. The altered molecules then get screened for disease-fighting abilities. Sometimes one tweak turns a bland compound into something with strong potential.
Dye and Material Synthesis
Colorants in plastics, textiles, and inks depend on precise chemical backbones for strong color and stability. 5-Chloro-2-fluoroaniline provides both. I’ve spoken to R&D specialists in synthetic dyes, and many highlight this compound’s balance of reactivity and selectivity. It enables the design of bright, durable colors that resist fading from light and washing. Some specialized coatings—which protect electronics or extend the lifespan of outdoor materials—make use of this chemistry. The result is a more reliable, longer-lasting end product for consumers.
Environmental and Safety Considerations
With specialty chemicals, there’s always the question of impact. The potential for runoff, mishandling, and accidental exposure means teams need strict protocols. Factories using 5-Chloro-2-fluoroaniline often invest in recycling solvent systems and train staff in safe handling. Sharing lessons from a plant visit, I saw how investing in fume hoods and modern filtration reduces workplace incidents and keeps emissions under control. Data from regulatory agencies shows that the most responsible firms not only do better at meeting environmental regulations, but they also improve risk management for their workers.
Building Trust Through Transparency and Research
People want to know how chemicals in their products are made and what risks exist. Brands using compounds like 5-Chloro-2-fluoroaniline support transparency by maintaining open access to safety data, independent risk assessments, and updates about ongoing research. Publicly available studies allow consumers and scientists to track any links to health or environmental concerns. Regulatory oversight helps too, making sure companies don’t cut corners or overstate safety. In practice, responsible makers of chemical ingredients earn trust by going beyond minimum legal requirements and sharing clear, honest information.
Looking Forward: Smarter Synthesis and Greener Chemistry
Chemists are experimenting with alternative methods that cut waste and energy use when making molecules like 5-Chloro-2-fluoroaniline. Green chemistry innovations focus on recyclable solvents, less toxic reagents, and using catalysts that speed up reactions without leaving unwanted byproducts. Improvements at the lab scale can eventually spill over into large-scale production, helping the whole supply chain. The people who invest their time and care in these advances are pushing the field toward safer, more sustainable solutions for everything from medicines to protective coatings.
Looking Out for Yourself and Others
People spend more time at the lab bench than they do reading guidelines, so knowing what to expect from 5-Chloro-2-fluoroaniline matters. It’s not the kind of chemical you want splashing on your skin or drifting toward your lungs. Handling aromatic amines, especially halogenated ones, often brings headaches or worse, and this one falls squarely in that category. Nobody wants a trip to the emergency room because they skipped goggles.
Exposure Risks: What to Watch For
Getting this stuff on your skin burns. Your lungs will take a hit if you breathe too much vapor or dust, because aromatic amines can damage tissue long before you notice. If it touches the eyes, damage kicks in fast. Some of the breakdown products show up in urine, meaning your kidneys also feel the impact.
Reports have connected long-term exposure to more serious concerns. There’s real fear about the links between some aniline derivatives and cancer. I’ve read studies in peer-reviewed journals—just a little extra exposure, over time, increases the risk. That memory sticks with you when you open a new bottle in the lab, so respect the dangers.
Safety Precautions: No Shortcuts
Chemical companies supply detailed safety data for a reason. Gloves made of nitrile get the job done, as latex lets smaller molecules right through. Long sleeves and a solid lab coat won’t stop everything, but they’ll keep a spill from reaching your skin, at least at first contact. Always use splash-proof goggles, not just cheap safety glasses.
A fume hood isn’t just a place to put your glassware: it’s where volatile, potentially toxic chemicals belong. The airflow keeps vapors out of your breathing space, which matters more than you’d think—many cases of hospital visits from anilines start with someone mixing or heating them outside a hood.
Wash your hands before eating or drinking; don’t treat the soap dispenser as optional. Label bottles and waste containers in big, bold letters. It prevents someone from grabbing the wrong thing during cleanup or disposal duty.
If Something Goes Wrong
Reading about exposure scenarios always hits home. If you spill 5-Chloro-2-fluoroaniline, flood the affected area with water for at least fifteen minutes and strip off any contaminated clothing. Ventilate the area right away. If it gets in the eyes, rinse at an eyewash station—don't hesitate and don’t try to wipe it out.
If symptoms start up—trouble breathing, burning eyes, skin rashes—get medical help fast. Don’t downplay what you’re feeling, even if nobody else seems worried. I’ve seen what happens when folks decide to tough it out, and it’s rarely pretty.
Managing Waste and Storage
Disposal isn’t a guessing game. Aromatic amines make wastewater dangerous and can’t simply go down the drain. Use airtight, labeled containers. Arrange pickup through a certified chemical disposal provider, not the regular custodial staff. For storage, keep it tightly sealed, away from strong acids, oxidizers, and sunlight.
Taking Responsibility
Lab work isn’t just about following reactions and taking measurements. Real trust comes from looking out for yourself and everyone around you. Handling chemicals like 5-Chloro-2-fluoroaniline calls for awareness, some humility, and constant respect for danger. Mistakes linger, both in memory and health, so doing things right the first time—every time—matters.
Understanding the Standard
No one wants to wager safety or quality on guesswork in the chemical business, especially with aromatic amines like 5-chloro-2-fluoroaniline. Chemists and procurement teams often look for a purity level of at least 98% when they buy this material. Accompanying documents typically list out impurities such as related anilines, moisture, and inorganic salts, and keep their levels below 2% in total. A specs sheet might mention GC or HPLC as the favored test (gas chromatography and high-performance liquid chromatography), both helping to lock down purity in a clear, repeatable way.
Why 98% Is the Trusted Figure
I’ve worked through audits and quality checks in the pharma world, and more often than not, we refused any lot below the 98% mark. Labs need tight consistency. Even a trace of extra impurity in a sensitive synthesis can grind everything to a halt. A bad batch means wasted time, and sometimes, thousands in lost revenue. Beyond that, small impurities sometimes give odd byproducts or reactions down the pipeline—nobody wants those surprises.
Why Vendors Aim for Consistency
Manufacturers don’t want their name attached to an inconsistent batch. Besides the obvious risk to reputation, pharma and agrochemical customers will walk away at the first whiff of trouble. Product recalls sting, and traceability means every gram gets tracked from start to finish. I’ve seen labs scrap entire lots because a single bottle missed purity spec. Meeting the 98% bar isn’t just about pride—it’s about survival in the market.
The Impact on Safety and Environment
Anyone who ever handled 5-chloro-2-fluoroaniline knows it isn’t baking soda. Proper purity matters for safety, not just product performance. Unaccounted-for impurities can interact in unpredictable ways, and sometimes they pack more toxicity than the main compound. Lowering unknowns in the drum lowers risk on the production line and in the warehouse. Chemical handlers, workers, and researchers get peace of mind knowing every bottle matches what the label claims.
Challenges in Hitting Purity Targets
Genuine 98% purity doesn’t come easy. During synthesis or distillation, side-reactions might slip in extra atoms where they aren’t wanted. Sometimes a byproduct shares similar physical properties with the main compound, making separation a headache. Labs often have to use extra steps—like recrystallization or advanced chromatography—to clean things up. That extra work boosts cost, but cutting corners risks compliance failures or rejections later.
How Quality Gets Verified
Most suppliers give buyers a certificate of analysis, with full testing results for purity, loss on drying, and impurity profile. Serious customers run their own incoming checks, sometimes cross-verifying with their own standards. During my time as a bench chemist, we caught out-of-spec shipments by running our own GC runs—nothing beat seeing the chromatogram with our own eyes. Trust matters, but so does verification.
What Can Improve the Process?
Better communication between supplier and user always helps. Customers with specific process needs should share those details early, so the supplier can hit the right spec without guessing. Stronger in-house analytics back up vendor claims. Finally, regular training at manufacturing sites lets staff catch issues before a drum leaves the plant. These steps cost a little more, but they keep surprises—and headaches—out of production schedules.
Bottom Line
Purity isn’t just a number for 5-chloro-2-fluoroaniline. It’s a guarantee the work ahead won’t result in wasted effort, extra risk, or regulatory hassle. Reputable vendors, good lab practice, and clear communication help everyone make the most of every batch.
Storing Chemicals Isn’t Guesswork
In any lab or warehouse, the way chemicals are handled can make or break safety. 5-Chloro-2-fluoroaniline is no lightweight oddity—it belongs to a class of aromatic amines, carrying with it certain risks and quirks. From my experience working around similar compounds, I’ve learned you can’t rely on old habits or shortcuts.
Why Conditions Matter for 5-Chloro-2-fluoroaniline
This compound gives off a sharp, chemical odor that serves as a fair warning: treat it with care. Like so many aniline derivatives, heat and moisture turn it unpredictable. Warm rooms speed up its degradation. Exposure to water, even just humid air, bumps up the risk of hydrolysis or leads to changes in purity.
I’ve seen colleagues get complacent. A forgotten bottle left open, or a leaky stopper on a shelf that’s a few degrees too warm—small things snowball. We once found a whole batch gone off in the summer. The product lost its color and utility, all because the A/C broke down for a couple of days. Financial loss and disposal headaches followed.
What Works: Dry, Cool, Sealed Up Tight
Setting the thermostat lower and investing in airtight storage are worth every penny. Temperature makes a difference, especially below 25°C. Any warm corner of a stockroom invites the kind of slow, invisible breakdown that only shows up when purity drops and experiments fail. Moisture-proof containers, such as amber glass bottles with PTFE-lined caps, put another barrier between the chemical and ambient humidity.
Keep these bottles away from direct sunlight. UV rays trigger chemical changes not always obvious until the material starts behaving strangely during synthesis. Light-proof cabinets or storage in dedicated chemical fridges give peace of mind. It doesn’t take high-tech solutions—just steady routines and the right placement.
Incompatible Compounds Bring Trouble
Storing 5-Chloro-2-fluoroaniline with acids or oxidizers is asking for trouble. The amine group can react with strong oxidizing agents, sometimes releasing heat or fumes. I’ve seen near misses when new hires stored it too close to nitric acid. Cross-checking storage plans with chemical compatibility charts isn’t bureaucracy; it’s basic self-preservation.
Labeling and separation save lives and material. Grouping with other aromatic amines (and away from acids and oxidizers) creates safer zones. Faulty shelving and careless stacking, on the other hand, make small spills hard to spot. A regular check of aisles, with fresh eyes looking for leaks or faded labels, should be part of any routine.
Personal Experience: Small Details Count
In labs I’ve managed, the simple habit of double-checking lids and storing chemicals two shelves above the ground prevents mishaps. It stops bottles from falling or staying unnoticed during cleaning rounds. Accidents often start with overlooked basics. Regular audits and encouraging staff to speak up at the first sign of something off go further than any manual.
For disposal, don’t pour any leftovers down the sink. Specialized containers (clearly marked and kept cool) keep waste under control till the next pickup. Even trace residues can threaten both crew and groundwater. Training new team members, not just handing them a safety sheet, builds the discipline needed to handle 5-Chloro-2-fluoroaniline right.
Committing to Best Practices
Following these storing tips isn’t some optional exercise. It’s about making sure labs run safely, products stay effective, and no one pays a price for small lapses. In chemical work, consistency isn’t just comfort—it’s how you keep promises to your team and whoever depends on your results.