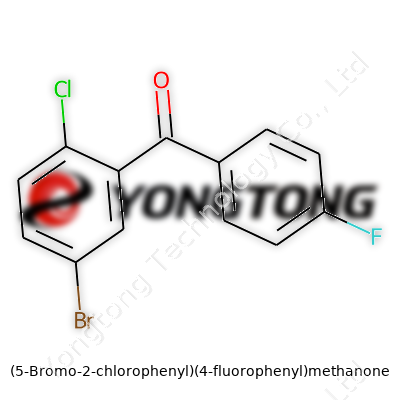
(5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone: A Closer Look
Historical Development
Chemists have pushed chemical synthesis far over the past century, stringing unusual molecules together to solve problems in medicine, material science, and agroscience. (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone’s development tracks alongside the rising demand for advanced building blocks used in ordered synthesis. Back in the late twentieth century, scientists looked for novel halogenated compounds that would yield better selectivity and reactivity in creating pharmaceuticals with exacting profiles. This compound emerged out of those efforts, combining several halogens on two aromatic rings in one molecule to bring new reactivity and specificity to downstream syntheses. As companies and research groups expanded high-throughput screening for drug candidates, they started using these multi-functionalized benzophenone structures with the dual goal of testing new chemistry and exploring biological action. Each round of innovation drew lessons from what stuck or failed in lab trials, and, as a result, the compound weathered several refinement cycles spanning three decades.
Product Overview
(5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone has carved out a spot in the labs working on medicinal chemistry and molecular design. The core structure features a ketone flanked by two aromatic rings, one bearing bromine and chlorine, the other sporting a fluorine atom. This set-up gives the compound characteristics useful both for reactivity and structure-activity studies. Many companies today provide it in high purity for scientists looking to test interactions at the molecular level, from electrophilic aromatic substitution to interactions with biologically relevant molecules. The compound’s complexity offers versatility: researchers value it not just as a chunk of carbon, hydrogen, and halogens but as a springboard to probe new synthetic spaces. Its use spreads outward into polymer precursor research and the design of ligands used in analytical chemistry, where halogen placement matters in supramolecular recognition.
Physical and Chemical Properties
What stands out with (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone is its solid, white-to-off-white powder form, generally stable at room temperature. Its melting point usually hovers between 105°C and 110°C, with a molecular weight of about 329.6 g/mol, thanks to the heavy halogen atoms. The compound’s low solubility in water contrasts with its ready dissolving in common organics like DMSO, DMF, and dichloromethane. These solubility traits affect which solvents and conditions work best during chemical reactions or purification. Chemists also recognize the electron-withdrawing tug from each halogen: the fluorine shrinks electron density, bending reactivity a certain way, and the larger bromine and chlorine atoms steer steric and electronic interactions, often making each reaction more predictable or more selective in the hands of an experienced chemist. Its ketone group anchors further modifications, letting chemists attach new moieties or explore reduction and alkylation pathways.
Technical Specifications & Labeling
Quality and consistency matter in research chemicals. Suppliers of (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone aim for purity above 98%. Labels include the compound name, batch number, manufacture and expiry dates, and storage instructions emphasizing keeping the bottle tightly sealed under desiccated and cool conditions. Many sellers issue a certificate of analysis, outlining elemental composition confirmed by NMR, IR, and mass spectrometry data, so labs know what they have in hand. Other information includes shelf life, appearance, molecular formula (C13H6BrClFO), and standard hazard pictograms reflecting its potential risk as an irritant or environmental hazard.
Preparation Method
The synthesis centers on Friedel–Crafts acylation. Chemists start with a solution of 5-bromo-2-chlorobenzoyl chloride and 4-fluorobenzene, using a Lewis acid like aluminum chloride to coax the molecules to couple. This exact sequence was hammered out by trial and error, balancing temperature, solvent choice, and reagent ratios—too much heat or not enough control, and byproducts pile up, reducing yield and purity. Many labs stick with dichloromethane or nitrobenzene as solvents, drawing on experience learned from making similar benzophenones. After workup and purification, especially column chromatography, the powder is dried and checked for unwanted impurities that might lurk from starting materials or catalyst leftovers. Advances in purification, particularly flash chromatography or recrystallization from ethanol, mean researchers today can count on product consistency lot by lot, a luxury early synthetic chemists rarely enjoyed.
Chemical Reactions & Modifications
This molecule’s design makes it perfect for further tinkering. Halogen atoms open doors for cross-coupling reactions so chemists can link on new aryl or alkyl groups using Suzuki, Heck, or Stille conditions. Common tricks include swapping in new groups by nucleophilic aromatic substitution or halogen–metal exchange, letting medicinal chemists fine-tune the electronic nature of the core scaffold to match research needs. The carbonyl group also offers leverage: it acts as a handle for reduction to build alcohol derivatives, or as a partner in condensation reactions to form larger frameworks that eventually serve as part of a drug’s backbone. Each alteration shifts both reactivity and biological relevance, so the research community has taken to using these patterns for iterative analog synthesis in drug discovery programs.
Synonyms & Product Names
Different labs and catalogs sometimes use alternate naming for clarity, traceability, or regulatory compliance. You might spot this chemical listed as 4'-Fluoro-5-bromo-2-chlorobenzophenone, or with synonym identifiers like (4-fluorophenyl)-(5-bromo-2-chlorophenyl)methanone. Some suppliers package it under research codes or catalog entries like BRCLF-KETONE or market it by its CAS number, making sure procurement follows the right document trail. These details help labs and companies avoid mix-ups and ensure the correct compound reaches the bench for research or development.
Safety & Operational Standards
Every laboratory using (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone institutes strict safety measures. Gloves, goggles, and fume hoods stay mandatory since skin absorption and inhalation both carry risks. Material Safety Data Sheets (MSDS) emphasize eye and skin irritation potential, the need for good ventilation, and the requirement for correct disposal, especially to avoid halogenated waste entering the environment. Some studies suggest caution during heating or scaling up reactions, given possible byproduct formation and toxicity. Labs now train staff on emergency measures, including spill containment and fire response, since the molecule, like many halogenated aromatics, can release hazardous fumes if burned. For environmental standards, effluents go through proper treatment systems.
Application Area
Chemists rely on this compound as both a synthetic intermediate and a model system for reaction design. Medicinal chemistry teams frequently use it to build novel kinase inhibitors, antitumor agents, and molecular probes, leveraging the halogen pattern to optimize how new molecules fit into protein binding sites. Pharmaceutical research often selects it to test structure-activity relationships, mapping how tiny tweaks change biological effects. Analytical chemists use it to calibrate detection instruments, while material scientists investigate its role in polymer precursor development and specialty coatings, since precise halogenation means better control over physical and chemical end properties. Outside the lab, these research outputs eventually inform treatments, new materials, agrochemicals, or regulatory reviews for environmental impact, evidence that foundation-phase chemistry underpins wide real-world advances.
Research & Development
Research groups worldwide have taken up (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone in hundreds of projects over the past decade. At university core labs, chemists screen it against enzyme and receptor panels as a fragment for structure-guided drug design. In industry, it features in combinatorial synthesis libraries, where thousands of similar compounds undergo parallel testing in search of a hit. The structure’s flexibility lets process chemists adjust protocols for better cost, safety, or yield, and over time, new methods, including microwave and flow chemistry, cut reaction times or reduce waste. Documented innovations from these efforts slip into journal articles, creating a virtuous cycle as new data prompt further improvements. Investment in research also spurs development of better analytical standards and reference compounds for regulatory-approved drug or chemical manufacturing.
Toxicity Research
Toxicological studies focus on two issues: the molecular structure’s halogenation and its potential to persist in biological systems. Though some halogenated aromatics have caused concern—think old school PCBs—this compound sits outside regulatory blacklists but still calls for careful evaluation. In cell line screens, high doses have shown cytotoxicity, suspected to arise from membrane interactions or reactivity with protein sites. Animal data remains sparse, so labs handle the molecule as potentially hazardous, tightening up labeling and restricting non-essential exposure. Longer-term effects on aquatic life have prompted researchers to test biodegradability and pathways for breakdown. Industrial users pay close attention, taking lessons learned from problems caused by careless halogenated waste disposal and applying them to containment and recycling routines. Hard evidence on chronic toxicity still accumulates, so every new toxicology and environmental study shapes future guidelines.
Future Prospects
The hunger for precise, tailorable intermediates will not vanish—if anything, molecular design continues to head for more complex, multifunctional frameworks, especially as AI-driven approaches map out new chemical spaces. (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone stands poised for more uses in targeted pharmaceutical assembly, as each added halogen or functional tweak helps uncover pathways to treatments with fewer side effects. Modern green chemistry now motivates researchers to find safer, less wasteful runs and improved end-of-life handling. With regulatory standards getting stricter year by year, producers and academic labs share results on safer syntheses and waste minimization, aiming to preserve utility while cutting hazards. As big data and automation filter down from tech giants to specialty chemistry, analytical workflows speed up insight cycles, letting research groups alter or discard approaches before issues ripple outward. In this landscape, the compound evolves from a mere molecular curiosity to a cornerstone tool for multidisciplinary breakthroughs, shaped as much by discovery as by lessons from past misuse and ambition.
Straight Talk on This Benzophenone Derivative
For years I’ve seen chemists light up at a new benzophenone derivative, mostly because these compounds show up in pharmaceuticals, dyes, and chemical research. There’s something fascinating about looking at a name like (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone and understanding what’s really going on at the atomic level. The chemical structure tells a story—a snapshot of how small changes in one ring or another can make a world of difference in how a molecule behaves. That rings especially true for this complex yet distinct compound.
Let’s break it down using real detail. The compound has two benzene rings. One ring carries three substituents: a bromine atom on carbon 5, a chlorine atom on carbon 2, and a carbonyl group connecting it to the partner ring. The other ring, simpler in comparison, carries a fluorine atom at position 4. The core that links these two rings is the “methanone” bridge, which is a carbonyl group (C=O) nestled between both aromatic systems. That carbonyl not only joins the rings but can make the compound a key intermediate for UV-absorbers or biological agents. You’d see this pattern—one aromatic group with two heavy halogens, another with a lighter fluorine—repeat itself in chemical libraries targeting activity screening.
Why the Structure Matters
People often ask if small changes in structure really change anything significant. That’s an easy one. Halogens like bromine, chlorine, and fluorine aren’t just decorations. Each brings something to the table. Bromine and chlorine both add bulk and electronic effects, making the original ring less reactive at certain positions. This can block unwanted side reactions in synthesis or affect how the whole molecule interacts with enzymes or materials. Fluorine stands out for its unique impact on electron distribution and resistance to metabolic breakdown, which is why big pharma uses it so much. More than that, the carbonyl moiety acts like a handle, ready for transformation or interaction with other molecules, driving function in research and commercial uses alike.
Over the years, I’ve watched how these seemingly simple tweaks on an aromatic ring reshape everything from chemical reactivity to regulatory approval. The structure here proves a classic point: rigorous attention to which atom goes where makes or breaks a candidate molecule. Chemists working on projects in photochemistry or medicinal chemistry care about the ultimate fate of that fluorinated benzene just as much as the bulkier bromo-chloro partner. It determines absorption properties, metabolic pathways, and even environmental persistence.
Challenges and Opportunities in Research and Application
Many hurdles show up during development, especially when heavy halogens enter the mix. Halogenated benzenes often challenge purification teams because they increase the difficulty of standard chromatography, and regulatory agencies take a sharper look at environmental impact in waste streams. Overcoming those headaches requires solid planning at bench level—thinking through greener synthesis strategies, tighter purification columns, and better documentation every step of the way. It always surprised me how, by focusing on selective reactivity and understanding the quirks of those halogen atoms, laboratories managed to carve out efficient synthetic routes.
Researchers get real gains when using detailed knowledge of structure—like in this compound—to predict physical behavior, improve yields, or design safer processes. Predictive modeling with quantum chemical tools cuts costs and wasted time, pushing innovation faster without trading off quality or safety. Smart teams put a premium on deep chemical insight, balancing creative synthesis with responsible development.
Knowing the structure of (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone sharpens every decision in the lab or on the production floor, proving just how much chemistry rests on the tiniest placement of atoms.
Why This Compound Matters
If you step into any synthetic organic chemistry lab, odd-sounding names like (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone start to make sense pretty quickly. This sort of molecule packs a set of halogens on its phenyl rings for a reason. The combination of bromine, chlorine, and fluorine in its structure means it acts as a foundation for building much more complex things. My time in research showed me: the heavier the halogen presence, the more places it goes in advanced drug discovery work and specialty materials.
Building Blocks in Pharmaceuticals
Nobody expects drug molecules to look simple. The medicinal chemists in my old lab would use molecules like this as scaffolds, altering the positions of those halogen atoms to affect biological activity. With its handle on electron distribution, this methanone core connects to proteins and enzymes differently than other templates. Sometimes, switches in halogen position unlock new anti-cancer or anti-inflammatory properties. It’s a guessing game, but you don’t win if you leave these building blocks on the sidelines.
A lot of modern cancer medicines and antivirals get their backbone from halogenated aromatic ketones. Scientists rely on this diversity during early-stage drug screening. Adding a fluorine or bromine opens or locks metabolic pathways, which keeps new medicines working longer in the body. Early studies suggest this compound’s derivatives help increase selectivity and stability during clinical trials.
Role in Agrochemical Innovation
Agriculture benefits from the same chemistry tricks used in drug discovery. Herbicides and fungicides often start with a halogenated phenyl core. A good friend of mine spent her postdoc trying to tweak herbicide selectivity. She showed me that substitutions like bromine and fluorine made her new candidates better at targeting weeds without harming the crops. (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone plays that supportive role. The distinct mix of atoms lets scientists tune water solubility and persistence in the field, reducing the need for repeated dosing.
Over the past decade, EPA databases have flagged the need for better breakdown in agrochemicals. Using fluorine or bromine helps products last through the season. Chemists have to balance efficiency and environmental persistence, as there’s strong demand for long-term, field-safe formulations. Innovations follow from the atomic choices made early on, right at this intermediate stage.
Stepping Beyond Health and Crop Science
These kinds of fluorine-rich templates sneak into specialty materials, too. Some high-performance polymers rely on similar aromatic ketones to hold up under heat or UV exposure. Engineers looking for rare qualities in electronics and coatings have turned to structures built from these halogenated building blocks. I learned this firsthand consulting with a company designing OLED display coatings – they needed just that combination of electron density and photostability.
It’s easy to overlook how these specialty molecules lay the groundwork for modern innovations. They pop up in patents as key intermediates. From new drug candidates to smart agriculture to electronics, chemists turn to these tools for good reasons.
Paths Forward and Responsible Chemistry
With all the excitement over what we can make, responsibility should stay in the picture. Regulatory bodies want better data on breakdown products and environmental impacts. Some researchers now focus on “greener” routes—using milder conditions or recyclable reagents. Less waste means cheaper production, and safer downstream handling cuts risks for us all. In my own work, cleaner syntheses opened collaborations and cut costs. The future for these intermediates hinges on that same blend of innovation and caution.
Why Chemical Safety Hits Close to Home
Anyone who’s spent time working in a lab, shop class, or even cleaning up with strong products will remember a time something stung, smelled strong, or gave them a headache. Chemical safety reaches a lot further than the workbench. People get hurt all the time by skipping gloves or ignoring symbols on bottles, thinking, “One splash won’t hurt.” Stories about burns, strange rashes, or scary fumes aren’t rare. Lax practices can roll into habits, and next thing you know, it’s a real emergency—not just for the person, but for anyone nearby. Trouble gets around quickly.
Practical Precautions to Respect
Labels aren’t decoration. They signal a warning for a reason. The hazard diamond, the splashy red and black “corrosive” sign—these mean something concrete: the risk is real. Gloves, goggles, lab coats, strong shoes, and masks do more than make you look serious. I remember running a demo for high schoolers, trying to clean up sulfuric acid. I thought a quick swipe with a rag would be enough. A stinging sensation on my fingertips lasted for days—a reminder that even minimal contact hurts. It’s tempting to skip protection, but it’s always a mistake.
Ventilation saves lives. Potent vapors don’t only smell bad, some knock you down. I’ve seen folks nearly black out in small spaces without fans or open windows. Make sure hoods or ventilators run. Windows can help in a pinch; working outside can be safest for small projects. Cleanups must use the right materials: plain water doesn’t always work. Some powders react and burn if water hits them. Know what absorbs, neutralizes, or contains the chemical you’re handling.
Never pour leftovers down a sink unless you’re sure it’s safe. Local rules might demand special disposal, and for a good reason—hazardous waste in the wrong place destroys pipes, leaches into soil, and poisons waterways. Once, in a university setting, improper disposal clogged drains and forced major repairs. It wasn’t just an inconvenience: the environmental crew spent weeks testing for contamination. Following rules prevents messy, expensive accidents.
Risks Don’t End When the Task Ends
Residual spills, splashes on skin, even fumes on your clothes can carry danger well after the job. Washing hands, changing clothes, and careful cleanup sound obvious, but far too many folks rush out and forget. Chemical containers need tight sealing and stashing somewhere secure—not within easy reach of children or unsuspecting staff. No one wants a surprise accident in a shared space.
Regular refresher training helps. Complacency settles in fast when you repeat tasks and haven’t seen an incident in a while. I’ve watched seasoned mechanics and researchers nod along to safety slides, only to catch them later skipping proper glove use. Sharing real-life stories, even scary examples, makes the risk feel immediate and personal. Familiarity breeds carelessness—reminders keep safety in focus.
Better Tools, Better Habits
Newer gear and products offer real improvements. Sensor alarms, safer packaging, spill kits—these aren’t just nice-to-haves. I’ve learned to trust good tools and to ask supervisors for updated versions if old equipment wears out or grows unreliable. It’s natural to get used to risk, but smart handling comes from the right habits. Teaching each other and staying alert is the surest path to protecting everyone in the shop, classroom, or lab.
Unpacking What Numbers Really Mean in the Lab
Across research labs and chemical plants, someone always asks, “What’s the molecular weight and purity of this product?” It sounds technical, but anyone who’s mixed baking powder into a recipe has stumbled into a version of the same idea. Take the molecular weight: that value tells you exactly how hefty a single molecule is. Knowing that isn’t limited to chemists with lab coats and goggles. Anyone dosing medicine, making fertilizer, or even brewing beer relies on these values, whether they realize it or not.
Molecular weight lays the foundation for everything else you do with a chemical. Anyone who’s had to dose out antibiotics at home probably felt a little nervous. Too much or too little, and things stop working as intended. The number itself comes from summing up all the atoms in the molecule. For example, a simple aspirin tablet needs a calculated dose, so accuracy matters. Doctors and pharmacists depend on those figures, but so do folks in agriculture, food processing, and even those working at craft breweries, since precise weights tie directly to efficacy and safety.
Chasing Purity: More Than Just a Fancy Label
Purity might sound more like a marketing buzzword, but it has teeth. Let’s say you’re making soap from scratch. Using pure lye instead of industrial-grade lye changes the outcome, from the soap’s smoothness to whether it irritates skin. In pharmaceuticals, even the smallest impurity can mean a world of difference. If contaminants creep into a batch of medication, people can end up in the hospital, or worse.
I’ve seen chemists cracking jokes about mystery powders and unexpected colors showing up in flasks. Those stories come from batches with low purity. That’s why every serious operation, from small research groups to big manufacturing companies, sets up rigorous purity tests. Methods like high-performance liquid chromatography and mass spectrometry show the world what’s hiding inside. These machines reveal low levels of byproducts, sneaky solvents left over from synthesis, and even dust kicked up by a clumsy lab technician.
Why People Should Care About These Numbers
As a student, I slipped up by using a reagent labeled “95% pure” in a reaction that demanded ultraclean conditions. My results went haywire, and the whole project stalled. That taught me to read every label, quiz suppliers, and demand certificates of analysis. These reports show molecular weight and exactly how much pure product you get. Practicing this at home feels like checking the nutrition label on food—except instead of salt or sugar, you’re looking for toxic side products or dangerous heavy metals.
There’s a public health angle, too. Over-the-counter vitamins, imported food additives, and even cleaning products often contain residues that aren’t listed. Regulations keep companies honest, and watchdog groups test products for what’s missing. As more products cross borders, transparency becomes a game of trust. People lean on numbers because suppliers sometimes overstate their purity claims.
Chasing Better Solutions in the Real World
Better labeling and robust third-party testing set a strong baseline. Labs that provide full traceability of batch records stand out above the rest. So does open communication: companies sharing safety data, analytical results, and anything unusual that pops up. It’s not just about ticking boxes for legal reasons. Informed customers raise standards and force manufacturers to act.
Pushing for education and better resources helps, too. Anyone buying chemicals, whether in quantities for craft projects or large-scale production, benefits from clear information. Safer reactions, accurate product claims, and peace of mind start with those values on the label.
The Importance of Smart Storage for Halogenated Ketones
Anyone working in a lab gets the routine: chemical properties drive how we store our inventory. (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone isn’t something you toss on any open shelf. This compound falls under the halogenated aromatic ketones family, carrying both reactivity and the sort of quirks that can turn a good experiment into a frustrating one if ignored. Years ago, I learned to respect chemicals like these after watching a costly, poorly stored batch yellow and lose purity. That mistake sticks with you.
Temperature and Light Both Matter
Room temperature means different things depending on where you work. In a climate-controlled facility, it might hover around 20°C; in a less modern building, swings of five degrees up or down can change everything. For (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone, I’ve only trusted dry, cool storage. Pushing temperatures above 25°C over long stretches turns the stability equation against you.
Light exposure does its own damage, given that aromatic halogen compounds don’t always hold steady under bright conditions. Once, a shipment sat near a sunny window and what was supposed to be bright white became off-color in half a week — a clear sign of degradation. Opaque, tightly sealed containers beat clear glass every time here.
Humidity: The Quiet Enemy
Moisture sneaks into any lab where storage protocols get lazy. Even trace water can eat away at the integrity of a halogenated ketone. In my years managing research inventories, desiccators became non-negotiable. I started using silica gel sachets for small sample jars and watched the number of ruined stocks drop. For bigger stores, a low-humidity storage room or chemical cabinet keeps things predictable. No sticky summer air means no clumping or mysterious chemical changes.
Keeping It Safe: Labeling and Segregation
Safety means not only knowing what sits in each jar, but making sure mistakes don’t happen at the shelf. I use both digital and handwritten logs. Labels never leave off the date and lot number. It’s tempting to tuck similar-looking bottles next to each other, but segregating halogenated substances avoids ugly surprises if any container leaks or off-gasses. Years ago, a mixed storage shelf led to corrosive vapors reacting with nearby oxidizers. That cleanup session took all afternoon and drove home just how quickly things can go wrong when shortcuts get taken.
Realistic Shelf Life
Manufacturers cite two to three years under the right conditions, but experience knocks that down. Small deviations pile up. I count on a practical shelf life closer to 18 months unless analysis proves otherwise. Regular QC checks matter; thin-layer chromatography and NMR confirm purity and catch changes before they cause headaches. No point in pushing your luck with expired materials — one failed reaction wastes time and resources.
Better Storage Is Safer and Cheaper
Keeping (5-Bromo-2-chlorophenyl)(4-fluorophenyl)methanone in top shape boils down to discipline: cool, dry, and dark storage; good labeling; consistent checks on purity. Smart storage keeps labs safer and cuts costs, since wasted batches add up quickly. Real experience says small practical changes make the difference between reliable experiments and costly mistakes.