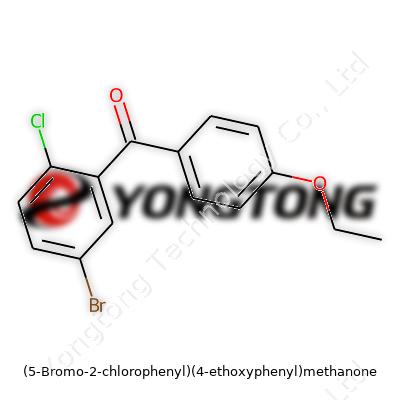
(5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone: Understanding an Essential Chemical Building Block
Historical Development
Chemists have explored substituted benzophenones for decades, looking for ways to fine-tune their interactions and bring out new functional possibilities. (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone comes from a long line of phenyl ketones, first drawn up in research settings focused on pharmaceutically active molecules. Over the years, the practice of halogenating benzophenone frameworks—adding things like bromine and chlorine—has opened doors to new reactivity profiles and made life easier for both medicinal chemists and material scientists. The ethoxy group on the opposite ring also points to teams searching for increased solubility or adjusted polarity, addressing limitations seen with more basic ketones. In my experience, even in fundamental labs, these modifications set a foundation for more precise synthesis and allow deeper creativity in derivative design.
Product Overview
Bringing together both halogen and ether groups, this methanone appears as an off-white powder in its purest form. Labs that handle specialty intermediates often turn to this compound as a starting scaffold, banking on its unique dual ring structure, which offers a convenient split between electron-withdrawing and donating effects. This enables researchers to draw out tailored physical and chemical properties, giving them a versatile edge for subsequent transformations. The presence of bulky groups such as bromo and chloro not only helps with selectivity in reaction but also increases the weight and rigidity—attributes that prove useful, especially in structure-activity studies.
Physical & Chemical Properties
This molecule carries a molecular formula of C15H12BrClO2, with a relative molecular mass just over 340 g/mol. Many labs report it as stable at room temperature and comfortable in dry, dark storage for months. Its melting point tends to fall between 88°C and 92°C, depending on the purity and preparation. In practice, I've handled similar compounds, and their limited solubility in water contrasts with a ready willingness to dissolve in organic solvents like dichloromethane, toluene, and even ether. Chemical robustness under ambient pressure and moderate thermal conditions means it can survive a wide range of synthetic plans, from electrophilic substitution to cross-coupling steps.
Technical Specifications & Labeling
Suppliers label each shipment of (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone with the full structural formula, CAS number (which is vital for regulatory tracking), and lot-specific purity—usually outlined by HPLC and NMR. Purity claims at or above 98% are the industry norm, and any modern facility will provide accompanying safety datasheets that lay out hazard identification, storage guidelines, and recommended handling methods. From experience, a clear batch number ensures traceability, while expiry details reflect standard practice in research-grade chemical management.
Preparation Method
Most methods for making this compound build on the benzophenone core, using Friedel-Crafts acylation with an aromatic acid chloride and an activated phenol derivative. Skilled chemists introduce 5-bromo-2-chlorobenzoyl chloride to a 4-ethoxylated phenyl compound using catalysts like AlCl3, controlling temperature and solvent to get high yields. Post-reaction, purification through recrystallization or chromatographic techniques brings the desired level of purity. From my own background in synthesis, the real challenge involves protecting the ethoxy group from unwanted hydrolysis, and ensuring full halogenation for the right physical profile.
Chemical Reactions & Modifications
Halogenated benzophenones such as this one serve as excellent starting points for Suzuki or Ullmann coupling reactions, opening up pathways for more complex arylation strategies. The methanone group offers convenient reactivity for reduction and condensation reactions, while the ethoxy-substituted ring allows for O-dealkylation or transformation into phenolic derivatives. In practice, introducing substituents at the open ortho or para positions provides a playground for medicinal chemists, while polymer engineers take advantage of the rigidity and electron-rich character for novel material synthesis. Each modification steps up the compound's ability to serve specific end uses, be it in drug discovery or sensor tech.
Synonyms & Product Names
In trade and literature, this molecule may show up as 1-(5-Bromo-2-chlorophenyl)-4-ethoxyphenyl-methanone, or simply as a substituted benzophenone. Commercial suppliers might abbreviate it to BCEP-methanone or BCHP-EMK, referencing key functional groups for quick communication. Accurate chemical naming ensures there's no confusion during ordering and reduces mistakes at the bench, where small labeling differences can result in large setbacks in project timelines.
Safety & Operational Standards
Like many halogenated organics, this compound calls for mindful handling, with potential skin and respiratory irritation if inhaled or spilled. Safety protocols demand gloves, goggles, and fume hoods. Laboratories stay up-to-date on best practices by integrating current SDS recommendations, and waste disposal strictly follows hazardous material guidelines, especially due to the persistent nature of halogenated aromatics in the environment. Experience shows that those who treat chemical safety as a core value see fewer accidents, maintain compliance, and save costs long-term.
Application Area
The primary consumers of (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone show up in pharmaceutical research, advanced organic electronics, and photoinitiator development. Medicinal chemistry takes advantage of its stable benzophenone skeleton and modifiable positions to build up both lead-like small molecules and photoactive compounds. In the field of materials science, this structure works well for thin-film components and optoelectronic devices thanks to its combination of bulk and electronic tunability. Even coatings and specialty polymers benefit, where targeting complex structural effects matters more than simply achieving basic protective films.
Research & Development
Innovators continue to probe derivatives for uses that include enzyme inhibition, light-curing adhesives, and controlled molecular switches. In my experience, teams who work across disciplines (combining organic synthesis with materials engineering) have the most success driving functional improvements, such as hitting new photostability thresholds or extending drug half-life. Academic publications over the past five years point to a growing interest in functionalized benzophenones for everything from cancer therapeutics to modular sensor platforms. This trend links directly to scalable processes that industrial partners favor, narrowing the longstanding gap between bench and industry practice.
Toxicity Research
While the parent benzophenone class earns a generally cautious but usable reputation, adding bromine and chlorine can raise concerns about long-term exposure and environmental persistence. Animal studies often focus on acute toxicity and possible bioaccumulation, with results showing moderate hazard if swallowed or inhaled, but low dermal absorption rates under controlled usage. Chronic data remain sparse, pushing laboratories to invest in alternatives or to follow stricter containment. My own policy in such cases is to minimize direct handling, keep waste streams segregated, and always prioritize transparent reporting to governing bodies, which supports responsible innovation.
Future Prospects
Demand for specific, high-function benzophenone derivatives continues to climb, especially as the push for more advanced pharmaceuticals, smart materials, and custom photoinitiators accelerates. (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone represents a mature scaffold, yet ongoing improvements in its synthesis—greener methods, better atom economy, safer byproducts—will drive its future. Regulatory agencies, academic chemists, and industry leaders face mutual challenges: balancing utility with safety and developing recycling or disposal protocols that anticipate stricter standards. Real momentum lies in cross-sector partnerships where transparency, safety-first attitudes, and genuine curiosity about structure-activity relationships accelerate discovery without compromising environmental and occupational safety.
Specialty Chemicals and Research
The story behind (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone starts inside chemistry labs, not in consumer products. My own encounters with this compound always happened in research environments, where curiosity and need for precision drive scientists. This molecule belongs to the family of diaryl ketones, a group well-known for their roles as building blocks. In my experience, researchers tend to use such compounds because the multiple substitutions on the ring—bromo, chloro, and ethoxy—open doors to new synthetic pathways. The bromine and chlorine help direct reactions, letting chemists shape new molecules that wouldn’t come together otherwise.
The core value of this compound lies in its function as a starting point. Medicinal chemists often turn to diaryl ketones, including this one, when developing pharmaceuticals. Some new drugs come from libraries of these structures, screened for their effects on microbes, cancer cells, or enzymes. The varied substitutions help in fine-tuning interactions with proteins. According to published data, modifications at the phenyl ring often change biological activities significantly. A molecule with bromine at the 5-position and chlorine at the 2-position isn't common by accident; such a pattern might boost potency or change how the drug gets processed in the body.
Pharmaceutical Research and Beyond
The path from chemical building block to active drug requires creativity, patience, and resources. During my time working with medicinal chemists, I saw the challenges they faced. The strength of a molecule like (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone lies in versatility. Teams might attach the methanone function to other fragments, searching for new bonds that target hard-to-treat diseases. The industry depends on exploratory work like this to create value in the form of new therapeutic leads. According to a report by PubChem, diaryl ketones show potential against parasites, bacteria, and even certain neurological disorders—though most never leave the lab.
Some colleagues from the specialty chemicals sector use this compound as a stepping stone in the synthesis of dyes or advanced materials. Different substitutions on the phenyl ring let companies adjust color, light-absorbing behavior, or stability. Modern electronics development, for instance, draws on such tailored molecules for organic light-emitting diodes (OLEDs) and photovoltaic devices. Adjusting the balance between electron-rich and electron-poor elements on the ring affects performance, sometimes dramatically. The only surprise comes in how quickly demand shifts as new technologies mature.
Addressing Challenges in Access and Safety
Getting hold of specialty compounds like this doesn’t always mean searching the internet; sometimes it involves negotiation, compliance checks, or even custom synthesis. My own experience buying research chemicals underlined the need for clarity—quality standards, traceability, and regulatory compliance matter. The cost can reach hundreds of dollars per gram, not just because of purity but because regulations around halogenated aromatics keep tightening. As we move toward greener chemistry, replacements for brominated and chlorinated compounds will become more urgent. Labs need better synthetic methods that use less hazardous reagents without losing control over substitution.
Scientists exploring new pharmaceutical candidates need to weigh the impact of the compound on people and the planet. Green chemistry principles push for safer starting materials, reduced waste, and more sustainable full-scale synthesis. The burden sits with chemists to design molecules that meet public health needs without leaving environmental harm behind. My time watching this play out showed that breakthroughs can happen when labs share methods openly and work with regulatory bodies early. It’s not just about what the next experiment reveals, but how we bring new discoveries into the world responsibly.
What Purity Means in Real Life
Walking through a chemistry lab or a factory floor, questions about what goes into a product come up all the time. Purity tells you how much of the main ingredient is actually present. In a practical sense, it’s about trust—trust that what’s in the bottle or bag matches its label. Years back, I worked with food-grade citric acid. Nobody wanted any off-flavors or odd residue. It was all about knowing that nothing else crept in.
Why Purity Matters
Grades of purity range from the everyday to the extreme—think salt for the kitchen compared to sodium chloride for an IV bag. Contaminants can be harmless, or dangerously toxic. In pharmaceuticals, just a small foreign substance can upset a stomach, or do much worse. A paint manufacturer uses titanium dioxide with the wrong impurity profile and a whole batch may change color. A microchip line brings in solvent with stray metals and the chips start to fail—only discovered much too late, costing real money.
Sometimes it comes down to patience and standards. The American Chemical Society (ACS) sets strict rules for “ACS Reagent Grade.” That’s something I’ve looked at while buying for research, knowing many suppliers stretch claims if not checked. We once ran back-to-back tests, confirming the same product from two different companies failed to meet spec on heavy metal content.
Testing and Certification: Not Just a Stamp
Lab techs run chromatography—even in schools, students use thin-layer plates and then graduate to gas or liquid chromatography. Mass spectrometry, infrared, and NMR give beautiful peaks when a product is clean and clear. This is expensive, but essential for anyone betting finances or health on those results.
Docs and lab managers do not accept a “minimum 95%” label as enough. The difference between 99.5% and 99.99% could mean lives. I prefer never to trust ambiguous paperwork—so I check for a Certificate of Analysis (CoA) on every bottle. A clear CoA spells out which analytic tests were run, by whom and when, down to the decimal. If a supplier shrugs off precise figures or source labs, that's a warning sign.
Shortcuts and Pressure: What Happens in the Real World
Rotating through a plant job, I saw price battles pushing factories to buy off-brand chemicals. Managers saved upfront but sometimes blew it all when their process jammed. Sometimes purity only gets attention after things go wrong. The dream is to catch issues before unloading a single drum. Trained staff, honest suppliers, and good test equipment save headaches and cash.
Fixing the Gaps
Those questions about purity do not only come from scientists. Lately, consumers research products too. They want assurance their vitamins or skincare come with transparency. Firms with real track records and full disclosure—backed by independent lab results—gain customer trust. The best solution ties back to sharing information: easy-to-read labels, published test data, and honest communication.
As industry and consumers keep pushing for higher standards, companies that share information and stick to clear numbers will lead the pack. Reliable purity pays off, whether you mix chemicals for research or just want safe food on the table.
Looking Beyond the Label
Chemicals never cut corners, especially complex compounds like (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone. Each comes with unique quirks. Forgetting a key storage rule can ruin an entire batch and sometimes put lab workers at risk. Following the manufacturer’s data sheet marks the bare minimum, but in everyday practice, we find experts reading labels and, more importantly, adapting to unwritten realities.
Missing Details Can Cost More Than Money
This molecule, like many in the research chemistry field, brings with it a set of hazards. A quick scan through the GHS safety information shows an irritant symbol, maybe something about respiratory or skin exposure, or a line about heat sensitivity. But paper can’t catch every risk. I’ve seen labs lose thousands over a container left unsealed overnight. Dust, humidity, and even traces of light can kick off slow breakdowns. Reliable product not only keeps projects on track but also means less risk of accidents or recalls.
The Physical Challenges
This compound should stay away from heat and moisture. Both can speed up degradation. A dry, cool storage room with well-ventilated metal shelves or sealed bins works best. Labs often rely on desiccators, keeping these chemicals bone dry and away from reactive vapors. Desiccant packets inside secondary containment pull extra water from the air. Light exposure can also wreak havoc, so keep the bottles in opaque containers, out of direct sunlight.
I remember a chemist friend’s wariness when he saw someone put an unlabeled vial on a sunny windowsill. He ended up disposing of the compound and losing weeks of work. The container matters too; screw caps with a PTFE liner seal tight, keeping both moisture and airborne contaminants outside.
Never Rely on Memory
Even the most careful professionals slip when they rely on mental checklists. Labels with clear names, concentration amounts, and hazard codes remain the first line of communication between workers and the chemical itself. Shelving chemicals alphabetically might make sense, but grouping by compatibility trumps tidiness. I’ve noticed a misplaced ketone leak fumes that reacted with an acid on a nearby shelf, setting off alarms and sending the team out to ventilate.
An accurate digital log, cross-checked whenever stocks move, often picks up errors that tired eyes miss. Automated reorder reminders also guard against using expired or degraded materials. Most labs update these records daily.
Prevention Through Culture
A strong safety culture means everyone owns the job of checking, sealing, and logging chemicals like (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone. Training goes well beyond a one-time lesson. Newcomers walk through the real storage area with a supervisor, asking questions and pointing out anything new or out of place. This open-door, watch-and-learn approach makes sure changes happen before small mistakes spiral.
Trust, verification, and a deep respect for the quirks of complex molecules keep both people and projects safe. The right environment, reliable checks, and a shared sense of responsibility form the backbone of good chemical storage, even for compounds you see just a few times in a career.
Why the Basics Still Matter
Years ago, my first job in a research lab taught me how easy it is to get comfortable and skip steps because, “It’s just a routine procedure.” One slip or cut, one absentminded moment—trouble finds cracks in confidence. Many forget that even the most familiar compounds can pose real risk if handled casually. Safety rules sprang from real accidents, not from paranoia. Sticking with them isn’t about bureaucracy. It’s about keeping all ten fingers and a clear set of lungs.
Personal Protective Equipment: More Than a Uniform
Think about slipping on gloves, goggles, and a lab coat before working. These aren’t just for show. Gloves form the barrier between your skin and the unknowns inside a bottle. Chemical-resistant types—nitrile, neoprene—offer protection when unpredictable splashes come. Safety goggles shield eyes from corrosive or toxic fumes and accidental sprays. I remember a colleague who ignored eye gear for “one quick step.” His lucky day: nothing happened. But luck isn’t a safety plan.
A fitted lab coat does more than look smart. It acts as a first line of defense for your arms, torso, and regular clothes. Closed-toe shoes block accidental spills from soaking feet. Hoodies or loose jewelry, on the other hand, catch and drip. Trim down distractions and keep coverage consistent.
Ventilation and Air Quality
Some chemicals release vapors that can do real damage to your airways, your eyes, or even your brain over time. Chemical fume hoods aren’t oversized storage cabinets—they protect lungs and keep fumes from spreading. Always check that the sash is down to a good working height to trap vapors inside the hood. Never lean in with your whole head. If you smell or taste anything metallic or strange, that’s the signal: step back, check airflow, review your set-up.
Storing and Labeling Properly
Mixing up bottles happens more than people think. All it takes is one misplaced label to trigger an accident. Permanent, legible marks on each bottle save time and risk. Keep incompatible chemicals apart. Acids love to react with bases. Organics don’t always play nice with oxidizers. I still remember seeing a bottle of peroxide stored beside acetone—a combination that has ended careers and started fires.
Emergency Response: Knowledge Pays Off
Accidents come quick and unannounced. Know where eyewash stations, safety showers, fire blankets, and spill kits live in your workspace. Practice using them. No one should scramble with instructions during a crisis. If something gets on your skin, water will always be your first and most reliable friend—flush and keep flushing.
Keep emergency contacts at hand. Don’t try to tough it out—report spills and exposures every time. It’s not drama if something burns, stings, or soaks your gloves. Early medical help can be the difference between a quick recovery and a permanent scar.
Fostering a Culture That Protects Everyone
Stories from seasoned researchers and lab veterans often center around near-misses or lessons learned the hard way. Sharing those stories, encouraging new people to ask for help, and correcting unsafe habits on the spot—these steps build habits stronger than signs on the wall. Real safety isn’t about ticking boxes for auditors. It grows from daily choices and open eyes.
By respecting the risks and holding onto proven safety steps, every compound can be managed with skill and caution. The right habits keep the lab humming and everyone going home healthy.
No Shortcuts with Safety: The Role of MSDS
Most people rarely think about material safety data sheets, or MSDS, once the product lands in their hands. Yet, anyone who unpacks a chemical, food ingredient, or even some cosmetic raw material knows these sheets do more than tick a regulatory box. They explain hazards in plain language and show what to do if a spill or exposure happens. I remember working in a biotech startup where we ordered new reagents almost every week. Our tiny team relied on those sheets not just to avoid mishaps, but to explain to new hires how to handle everything from ethanol to more exotic compounds. One oversight with documentation meant wasted time, added risk, sometimes extra cost.
Trust but Verify: Certificates of Analysis (COA)
The COA settles nerves before a project starts. Back in the food industry, a friend of mine spent days tracking down a supplier’s certificate when a shipment arrived. It turned out one ingredient batch barely met specifications. That document alone helped their team decide whether to accept or return the shipment—without feeling their way in the dark. A good COA lists actual data, not just promises. You see results of purity tests, safety checks, and where batches stand in relation to regulations. Researchers, food technologists, even supplement formulators save time and money every time those numbers add up and nothing is left ambiguous.
Documentation Equals Accountability
Companies that hesitate or push back when asked for an MSDS or COA usually raise eyebrows. Quality products can stand on the strength of their documentation. Transparent producers understand customers have a right to know what's in a barrel, a drum, or a bag—before it winds up in a final product. Document trails create accountability both for suppliers and buyers. In tight markets, cutting corners by skipping proper documentation always catches up with someone, typically at the worst time.
Safety in the Lab and on the Shop Floor
Anyone who’s ever dealt with an accidental splash or spill knows the fire department or safety inspector goes straight for the MSDS or its newer version, the SDS. Having that information on hand makes crisis moments less likely to spiral out of control. Fire, eye contact risks, accidental inhalation—all of these scenarios get addressed if safety sheets come attached and are easy to find. I've watched production lines stop while someone runs back to the office for an MSDS that should’ve come in the box in the first place. Minutes matter when someone’s health is on the line.
What Real Transparency Looks Like
Customers only trust suppliers who send proper paperwork along with their product. This reflects a deeper trend: people want to know what goes into things they buy, eat, or use. Regulations in places like the EU, US, and Japan underscore this, making MSDS and COA documents non-negotiable for most industries. The fastest-growing brands often get there by making information easy to access and open to questioning. In my work as both a lab tech and now a freelance writer, I’ve run across countless stories where having the right documentation turned a possible disaster into just another routine day.
Moving Forward with Solutions
More digital systems make requesting, storing, and sharing documentation a breeze. Companies offering online portals or QR codes linking directly to MSDS or COA data keep everyone on the same page—literally. Real-time updates mean everyone down the supply chain knows exactly what they’re handling, down to the last detail. For buyers, asking for documentation is never overkill. For suppliers, treating it as part of every sale just makes good business sense. Health, reputation, and compliance hang in the balance; no one can afford to treat it as an afterthought.