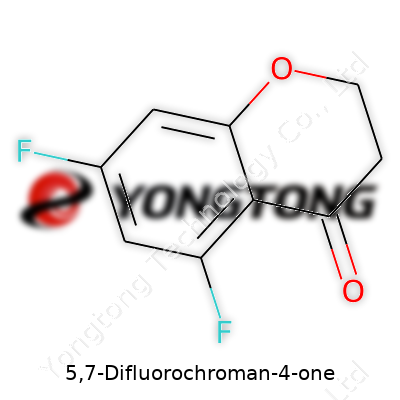
5,7-Difluorochroman-4-one: Exploring Its Role, Properties, and Potential
Historical Development
Fluorinated chromanones caught the attention of chemists in the late twentieth century, driven by the hunt for new pharmaceuticals and agrochemicals. Over the years, researchers noticed promising features in the chroman-4-one skeleton and began to tweak its structure, sliding fluorine atoms into their framework. The drive to introduce electron-withdrawing fluorine atoms comes from their ability to change metabolic stability, bioavailability, and overall reactivity—a coveted trait for those looking to boost compound lifespans or biological punch. By the late 1990s and early 2000s, advances in organofluorine chemistry and the refinement of fluorination methods made compounds like 5,7-difluorochroman-4-one easier to synthesize and study. Synthetic fluoroaromatics rode a wave of innovation as more labs saw their potential and demanded better access to pure, consistent supplies. This journey marks 5,7-difluorochroman-4-one as both a result of creative chemistry and as a stepping stone for new molecular designs in research and industry.
Product Overview
5,7-Difluorochroman-4-one falls within the class of chromanones—molecules featuring a benzopyranone core—decorated with fluorine atoms at the 5 and 7 positions of the benzene ring. The compound serves as an intermediate in pharmaceutical synthesis and as a building block for crafting specialty chemicals. Demand for such structures springs from their ability to introduce new functions with minimal structural changes, shaping bioactivity in subtle but powerful ways. With both aromatic and aliphatic features, this compound bridges traditional and modern chemistry, becoming a frequent guest in medicinal chemistry labs aiming to boost target engagement or lower off-target effects.
Physical & Chemical Properties
The inclusion of two fluorine atoms on the aromatic ring lends 5,7-difluorochroman-4-one increased lipophilicity and higher metabolic stability, compared to its non-fluorinated cousins. Solid at room temperature, the substance typically presents as an off-white to pale-yellow powder, often crystalline, signaling purity and proper storage. Boiling and melting points tend to outstrip those of non-fluorinated chromanones, sometimes hitting values above 200°C for melting and higher for boiling, depending on sample handling and purity. Solubility stays high in organic solvents like dichloromethane, chloroform, and DMSO, though water solubility drops, a common pattern for many fluorinated aromatics. The molecule shrugs off mild acids and bases in its neutral form but responds to stronger conditions. Its chemical backbone remains stable under ambient conditions, but stems from its reactivity toward nucleophiles or during oxidation, making it a collaborative partner in synthetic campaigns.
Technical Specifications & Labeling
Labs and manufacturers typically offer 5,7-difluorochroman-4-one in sample vials or bulk containers, depending on research demand. Labeling always highlights purity, with high-performance liquid chromatography (HPLC) readings frequently topping 97%. Lot numbers trace back production details. The label displays molecular formula (C9H4F2O2), molecular weight (182.13 g/mol), and structural identifiers, including CAS number and, at times, a QR code pointing to detailed certifications and material safety data. Batches undergo identity checks using NMR spectroscopy and mass spectrometry, ensuring traceability and reproducibility in downstream applications. Storage temperature, humidity recommendations, and hazard warnings follow, often spelling out that direct skin or inhalation contact may pose health risks given the compound’s synthetic origin and lack of thorough toxicology data.
Preparation Method
Chemical synthesis of 5,7-difluorochroman-4-one generally starts with a suitably substituted resorcinol compound, carrying fluoro groups at positions intended for the final target. Friedel–Crafts acylation lays down the chromanone core, often using an acid chloride and Lewis acid catalyst to drive cyclization. The reaction requires vigilance; even minor fluctuations in temperature or stoichiometry can tip the scales toward undesired byproducts. Following cyclization, purification happens using silica gel column chromatography, yielding the product in millimolar to multigram quantities. This step draws from long years of fine-tuning in synthetic organic chemistry, harnessing solvent gradients to coax the desired compound out with high purity. Advances in fluoride-selective fluorination techniques have opened the door to later-stage modification, a strategy favored when late-stage functionalization promises better yields or more streamlined workups.
Chemical Reactions & Modifications
Applied chemists prize 5,7-difluorochroman-4-one for its amenability to further chemistry. The electron-deficient aromatic ring, tricked out with fluorines, becomes less reactive to electrophilic substitution but opens up other doors—such as nucleophilic aromatic substitution, especially at positions next to the fluorinated carbons. Reduction of the ketone group delivers chromanol analogs, opening pharmacological possibilities, while oxidation or halogenation brings new faces to the molecule, sometimes forming precursors for more advanced drug-like compounds. Coupling reactions introduce aryl or alkyl chains at peri-positions, giving medicinal chemists a way to expand molecular diversity quickly. Conjugation strategies, such as forming esters or amides, unlock library synthesis for drug screening. Each reaction builds on reliable fundamentals, yet requires keen awareness of the impact each structural change brings.
Synonyms & Product Names
The compound crops up under several names in catalogs and literature. Common synonyms include 5,7-difluoro-2,3-dihydro-4H-1-benzopyran-4-one and 5,7-difluorochromone. Suppliers may use product codes or blend in proprietary trade names—these rarely stick in academic settings but help buyers confirm identity across different producers. Chemists lean toward structural descriptors, given their transparency and universal recognition. CAS number 672-65-1 frequently serves as a dependable touchpoint, more universal than branding or vernacular.
Safety & Operational Standards
Personal experience and anecdotes from countless labs teach that handling fluorinated aromatics requires vigilance. Accidental spills release dust that stings the eyes and tickles airways. Standard operating protocols call for nitrile gloves, proper eyewear, and fume hoods, particularly during weighing, dissolving, or transferring the compound. Ventilated storage away from acids or bases reduces accident risks. Disposal routes channel such chemicals toward certified hazardous waste streams, never down the drain. Many regulatory agencies still lack specific guidelines for this compound due to the gap in toxicity data, so researchers lean on broader safety frameworks for synthetic organics—erring on the side of caution and keenly documenting every exposure or incident.
Application Area
Medicinal chemistry dominates the application landscape. The molecule slips into the synthesis of candidate pharmaceuticals, especially those chasing heightened metabolic stability. Preclinical work draws on its interaction with biological membranes and potential for enzyme inhibition, with some derivatives tested for antifungal, antiviral, and anticancer profiles. Agrochemical firms evaluate similar structures as possible fungicides or plant protectants, tapping the compound’s ability to resist biodegradation in the field. For material science, the chromanone core can serve as a stepping stone to more complex polymers or functionalized coatings, the fluorine atoms improving weatherability or chemical resistance. Academic labs covet the molecule for its role in structure-activity relationship (SAR) studies, helping dissect the web of interactions underpinning small molecule action in nature and medicine.
Research & Development
Active research continues to examine just how much the twin fluorine atoms bend biological outcomes. Scientists screen the parent compound and its analogs across enzyme panels, cell lines, and animal models. Projects target both the parent molecule and libraries spun from quick modification of the chromanone core, using modern parallel synthesis tools to churn out hundreds of molecules for testing in a single week. Key journals report advances in optimizing reaction selectivity or in developing greener pathways that reduce waste from halogenation steps. Pharmaceutical R&D programs frequently seed new projects with this motif, hoping to leap over metabolic liability hurdles that hamper lead development. Many hope to discover whether the compound’s intrinsic stability confers unique pharmacokinetic features or improved activity for chronic medications.
Toxicity Research
Despite growing use, toxicological profiles remain patchy. Initial findings suggest low acute toxicity for parent chromanones, but fluorinated aromatics demand fresh scrutiny due to their stubborn persistence in biological systems and the environment. Animal studies, when available, suggest that metabolism might proceed more slowly, raising questions about bioaccumulation. Chronic exposure data remain rare, pushing researchers to advocate for rigorous assessment before any wide adoption outside the lab. Industrial safety data sheets stress minimizing contact and always using personal protective equipment until full toxicokinetic data emerges. Regulatory frameworks continue to evolve as new studies trickle in, gradually filling gaps in understanding and shaping future guidelines for researchers and end users.
Future Prospects
The search for more effective and stable compounds in both medicine and agriculture continues to fuel interest in 5,7-difluorochroman-4-one. Synthetic chemists look for faster, cleaner, and economically scalable routes, aiming to meet demand while trimming waste. Medicinal chemists watch for new links between structure and function that might push this scaffold into clinical trials. Environmental chemists keep a sharp eye on persistence and breakdown patterns, navigating the delicate balance between utility and environmental responsibility. Advances in computational chemistry and high-throughput screening promise to crack open more secrets tucked inside this molecule’s frame, leading to more tailored modifications and potentially safer, greener options. Future research may also unlock uses in materials science and advanced coatings, where the push for durability lines up nicely with the features fluorine atoms deliver on this distinctive compound.
Digging into 5,7-Difluorochroman-4-one
5,7-Difluorochroman-4-one stands out among chromanone derivatives, thanks to clever tweaks to the typical chromone skeleton. Swapping out hydrogen atoms for fluorines at positions five and seven isn’t just an arbitrary change—tiny modifications like this can flip the script on how a compound behaves, both in a test tube and inside a living system. In the chemical world, that spells fresh possibilities for drug development and molecular research.
Breaking Down the Chemical Structure
Let’s imagine the backbone: there’s the chromanone ring, which means you’re looking at a fused ring system. One part is a benzene ring, and tucked against it, you’ll find a six-membered oxygen-containing pyran ring. The “4-one” means there’s a ketone sticking off carbon number four. Stick fluorine atoms on carbons five and seven—right on the aromatic (benzene) portion. This sets the structure apart in how it balances electron flow, responds to chemical reactions, and resists biological breakdown.
For clarity, here’s what you’d see drawing it on paper: a benzene ring with two fluorines in the five and seven spots, merged with a six-membered ring that holds an oxygen and a ketone. Chemical shorthand looks like C9H4F2O2. Small differences in where these fluorines sit translate into big shifts in reactivity. With a molecular structure like this, activity in the lab can shift in wild and unpredictable ways, compared with a plain chromone.
Why Structure Tweaks Matter
Swapping hydrogens for fluorine atoms isn’t just about switching up a few letters in a formula. In experience, fluorine can hustle its way into a molecule and make it more rugged. Enzymes find it tougher to break apart, which matters if you’re designing a drug you want to last longer in the body. These modifications can also change how the molecule slots into biological targets—sometimes improving potency or tweaking the pattern of side effects.
The two fluorines on 5,7-Difluorochroman-4-one drop the electron density around the benzene ring, often translating to different biological profiles. In research circles, these compounds can show higher stability, better membrane crossing, and even unique binding patterns to protein targets. Real-life applications have included chasing after new antibiotic leads, as well as searching for molecules that might block cancer cell growth or tweak the brain’s signaling chemistry.
Tackling Synthesis and Research Challenges
Chemists meet challenges trying to prepare molecules like 5,7-Difluorochroman-4-one, mostly because selectively dropping in those fluorines takes some finesse. Years spent in a university lab taught the importance of methodical planning: a tiny mistake during synthesis can scramble where those fluorines land, leading to a jumble that’s tough to untangle during purification. These aren’t just problems in theory—they’re headaches that eat up budgets and precious hours in the lab.
Cutting-edge approaches include using specialized catalysts and reagents that can guide fluorination more precisely, shrinking the risk of side products. Lab teams who combine old-school knowhow with new technology often find success faster, keeping toxic byproducts in check and boosting overall yields. Cleaner processes help labs stick to health and environmental safety standards—absolutely crucial when moving toward scaling up production.
Looking Forward
Paying close attention to the chemical structure of 5,7-Difluorochroman-4-one helps researchers shape better experiments and dream up applications with real-world impact, whether in therapy, diagnostic tools, or agricultural chemistry. Every atom and bond really does matter, and smart structural choices lead to discoveries strong enough to stand the test of time and scrutiny.
Why Chemists Keep Their Eyes on 5,7-Difluorochroman-4-one
Anyone who has spent time around a chemical synthesis lab knows simple molecules can lead to complex possibilities. 5,7-Difluorochroman-4-one gives chemists a flexible starting point, and you see its fingerprints across chemical research. Its backbone stands out because those two fluorine atoms change the game. They tweak the molecule’s stability and how it interacts in the body. I’ve seen researchers spend weeks looking for just the right building block, and this compound gets a lot of attention for a reason.
The Role in Drug Discovery
In drug development, researchers often run into molecules that break down too quickly in the human body. Two fluorine atoms on 5,7-Difluorochroman-4-one help keep it from falling apart, letting it last longer. Drug companies often search for molecules with a chromanone core like this one because of its connection to antioxidants and enzyme inhibitors. Some research teams use it as a scaffold to build on, experimenting with different chemical groups to turn it into something that blocks unwanted enzymes. You’ll find it in plenty of studies tied to neuroprotection and cancer-fighting molecules. Because of its versatility, it pops up in academic papers where new drug candidates start their journey.
Stepping Into Agrochemicals
Farmers and agronomists keep a close eye on new agrochemicals. Many crop protection agents trace their roots back to fluorinated compounds like 5,7-Difluorochroman-4-one. Its structure lets scientists craft new insecticides that resist breakdown in the field. I remember talking to a peer who tested modified chromanone derivatives on wheat rust—yield protection jumped in plots where these molecules had been introduced. Researchers know that tweaking molecules for longer field life and lower toxicity often starts with a versatile fluorinated backbone.
Material Science Gets Involved
Electronics companies are always searching for stable, high-performance materials. Some versions of chromanone derivatives find their way into organic light-emitting diodes (OLEDs) and advanced coatings. The fluorines on 5,7-Difluorochroman-4-one give it better thermal stability and alter how it conducts electricity. I’ve read papers where researchers used this molecule as a precursor for specialty polymers—products that need to handle extremes in heat or electricity. These materials often show up in consumer gadgets, electric vehicle displays, or as parts of solar panels.
Challenges and Options Moving Forward
No molecule brings only upside. Synthesizing difluorinated compounds can cost more and sometimes delivers waste that needs special handling. Not everyone can afford the high cost of these building blocks, making wide-scale adoption tough in smaller markets. Many researchers look for greener methods to make these compounds. Some labs have started experimenting with biocatalysis to produce similar chromanone derivatives, reducing hazardous byproducts. Academic and industry partnerships make a difference when it comes to finding safer and more efficient paths from raw material to final application.
People outside the research world might not hear about 5,7-Difluorochroman-4-one by name, but the real impact comes when its applications improve food security, health treatments, or electronics. Whether it’s about keeping crops healthy, making new medicines last longer, or helping devices run cooler, this fluorinated chromanone keeps showing up where progress matters.
The Role of Molecular Weight in Chemistry and Industry
Molecular weight isn’t just a detail on a data sheet; it shapes how chemists and manufacturers work with molecules every day. In research labs, accuracy matters. One decimal in the wrong place throws off calculations and experiments. Molecular weight tells us how much of a compound to weigh out, how compounds behave in mixtures, and how drugs travel through the body. Looking at 5,7-Difluorochroman-4-one, precision in understanding its molecular makeup forms the foundation for useful results across different applications.
Calculating 5,7-Difluorochroman-4-one’s Molecular Weight
5,7-Difluorochroman-4-one carries the chemical formula C9H4F2O2. Each atom inside has a known atomic weight: carbon gives 12.01 g/mol, hydrogen is 1.01 g/mol, fluorine hits 19.00 g/mol, and oxygen settles at 16.00 g/mol. A quick calculation looks like this:
- 9 carbons (9 × 12.01) = 108.09
- 4 hydrogens (4 × 1.01) = 4.04
- 2 fluorines (2 × 19.00) = 38.00
- 2 oxygens (2 × 16.00) = 32.00
Add those values and the answer lands at 182.13 g/mol. That number isn’t just trivia—it drives the way chemists approach everything from simple reactions to building blocks for materials.
Why This Number Really Matters
Every major lab purchase, every reaction mixture, even safety calculations in the workplace look back to the molecular weight. Imagine a drug manufacturer trying to scale up a pilot project. If the team misjudges the molecular weight by even a few grams, the error snowballs down the line, driving up costs and wasting resources. In biological systems, the wrong weight gives faulty dose calculations and impacts research reproducibility. Environmental research often uses molecular weight as a reference point for tracking contaminants—thinking of pesticides, fluorinated compounds, or pharmaceuticals leaking into waterways.
A mistake in the numbers doesn’t just frustrate technicians—it bends research outcomes or creates risk in production environments. By keeping molecular weight front and center, researchers avoid costly setbacks and deliver better, safer results.
Building Good Practice Around Chemical Information
Reliable chemistry means treating the small details with respect. Getting the molecular weight right starts at the source—triple-checking formulas, consulting reputable databases such as PubChem or ChemSpider, and cross-referencing physical samples against paperwork. Teams who care about their data leave less room for slipups and confusion, especially under deadlines.
In my own time working with analytical standards, every step from sample labeling to measurement was double-checked. Mismatched molecular weights weren’t rare, especially with complex fluorinated compounds. A habit grew around re-verifying numbers before sharing results. It saved more time than it cost.
Chemists and engineers share responsibilities here. By championing accuracy, standard operating procedures, and open communication, teams steer clear of the pitfalls hidden in bad calculations.
Low-Tech Solutions for High-Stakes Numbers
A simple calculator, periodic table, and a well-maintained lab book still solve the problem. Modern labs can take this further with digital tools, but the process itself stays humble—add the atoms, compare the answer, don’t skip steps. The best solutions often come from building habits around double-checking and documenting each answer as if someone else has to read it cold.
Molecular weight might seem like a small fact, but in the world of chemicals like 5,7-Difluorochroman-4-one, it’s the key that unlocks reliable results all along the chain from test tube to real-world impact.
Respecting Chemicals Beyond the Basics
Working with lab chemicals, especially ones containing fluorine, gets personal fast. After a few close calls with corrosive splashes years ago, I pay extra attention to safety data sheets—and 5,7-Difluorochroman-4-one isn’t just a tongue-twister; it’s the kind of compound that expects full respect. That fluorine migration shows up in reactivity and toxicity, making easygoing handling a big mistake.
Solid Ground: Personal Protective Equipment
Gloves count for more than compliance—here, nitrile or heavy-duty neoprene forms a real barrier. Dust or powder off one’s bare skin spells trouble, as fluorinated rings tend to stick around and resist simple clean-up. A lab coat, buttoned, prevents accidental drips from reaching clothes, which helps later when changing for home. Most chemists I know skip exposed ankles; safety goggles cover wide sides on your face. Half-measures create risk.
Air Quality, Not Just Clean Hands
Fume hood use goes from “strong suggestion” to “non-negotiable” with aromatic fluorinated ketones. Even moderate volatility gets risky fast: one minute without draft protection, and that musty note indicates airborne compound on the move. Fluorinated molecules don’t play by the rules of less reactive aromatics. Every laboratory accident report with similar compounds shouts this lesson: run those exhausts, test airflow with tissue, and use vented waste containers to stop vapor build-up.
Mixing and Measuring Mean Patience
Good technique wins over speed. Powder sticks to spatulas in unpredictable ways, and static draws up dust clouds that cling to sleeves or wrists. Many seasoned technicians turn to antistatic mats, slow steady scooping, and gentle weighing. Rushed actions lift dust, and even microgram traces of reactive fluorine-charged droplets trigger irritation or, with enough exposure, heavy symptoms much later.
Waste Handling as a Priority
Old habits like pouring any unused substance down the drain absolutely backfire. Sitting in a university lab years back, I saw accidents happen from “safe” sink disposal, which mixed fluorinated substances with acids or bases in pipes. That creates corrosive byproducts. Dedicated fluorinated-organic waste bottles cut this risk. Every drop or contaminated wipe, no matter how small, belongs in the proper bin, sealed tight and labeled so no one else pays for carelessness on someone else’s shift.
What Instructors and Supervisors Should Share
Training never finishes. Institutions can plug knowledge gaps with walkthrough demos—real goggles, real gloves, with all the proper labeling and waste tracking. A peer mentor sharing scars and near-misses reaches far past mandatory videos. Posters at every relevant bench catch eyes, and expiry logs remind people to check the integrity of bottles and room systems.
Building a Culture of Care
Fluorinated ring compounds offer immense value in materials and pharmaceuticals, but a healthy respect always outweighs overconfidence. Investigating incidents reveals stories of distraction or corner-cutting. My own practice changed once I realized safety isn’t a set of isolated rules—it’s daily habit, double-checks, clear conversations, and never, ever taking eye protection for granted.
Why Storage Matters
Safety takes priority anytime chemicals enter the picture. Working with 5,7-Difluorochroman-4-one doesn’t differ. I’ve seen what happens when folks overlook proper storage—chemicals degrade, accidents pop up, research gets derailed. Detail-oriented storage isn’t extra work; it protects people and preserves the chemical’s integrity.
Understanding 5,7-Difluorochroman-4-one's Nature
This compound features two fluorine atoms, giving it a unique set of properties. Fluorinated aromatics resist moisture better than many organics, but that doesn’t mean they’re indestructible. Exposure to humidity, heat, and light can shift stability or lead to slow decomposition.
Room Temperature Won’t Cut It
Standard room shelves rarely do justice to specialty reagents. I prefer storing sensitive aromatics in a cool, dry, and well-ventilated area. Long-term, sealed amber bottles prevent light from triggering side reactions. I use desiccators with fresh desiccant packs to fight off any humidity sneaking into containers, especially during stormy seasons. Dry air keeps mold and hydrolysis in check, which matters for both lab safety and research accuracy.
Cleanliness Removes Risks
Cross-contamination doesn’t always look dramatic—sometimes the culprits involve traces of acids or bases left behind from old samples. My habit: I always use clearly labeled, dedicated containers. Gloves might seem obvious, but too many times I’ve watched others skip them while handling bottles. Sweat and skin oils can slip past unnoticed, damaging both the reagent and the handler.
Shelf Life—Not Just a Theory
Manufacturers give expiration dates for a reason. Even chemicals with a tough reputation will lose potency or pick up impurities after years sitting around. I run regular inventory checks, rotating fresh stock forward and safely disposing of anything that looks questionable—or has hit its expiration window. GHS labels help track age and hazard class. My own system includes using colored dots to mark “open since” dates.
Emergency Preparedness
Spills happen, no matter how careful you are. I keep spill kits nearby, complete with activated carbon, gloves, goggles, and a chemical waste container. I’ve sat through enough safety meetings to know that reading the SDS sheet isn’t a box-checking exercise—it tells you what to do if things go south. NIOSH recommends local exhaust ventilation when handling powders. I keep the fume hood sash low and power running.
Solutions Start with Routine
Safe storage isn’t just about following a checklist. Building habits around safety makes a difference: double-checking caps, logging each use, and placing chemicals with similar hazards together—never tossing everything onto a single shelf. Peer review helps, too. Everyone in my lab gets a walk-through on new reagents, and senior researchers run surprise audits to catch bottlenecks or sloppy habits before they create problems.
Protecting People and Results
Proper care of chemicals like 5,7-Difluorochroman-4-one means fewer headaches for researchers and less risk for support staff. Good storage practices preserve both safety and science—no shortcuts, no guesswork, just consistent attention to detail.