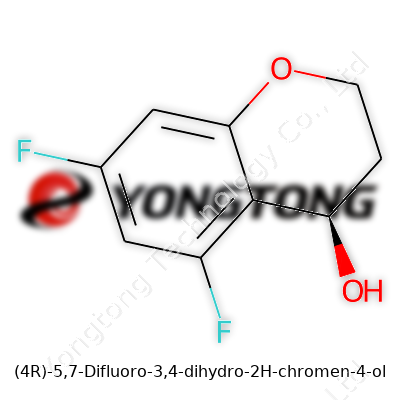
(4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol: A Deep Dive Across Science and Innovation
Historical Development
The story of (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol connects with the stride of fluorine chemistry throughout the twentieth century. Early research on chromenes centered on their occurrence in natural products, especially for the promise they showed in pharmaceuticals and crop protection. Scientists working in the late 1900s tackled the challenge of introducing specific fluorine atoms onto aromatic systems, chasing activity improvements. Fluorinated chromenes captured the curiosity of both industrial and academic labs, their unique physiochemical profile leading them into drug discovery efforts. The refinement of regioselective fluorination, more predictable synthetic tools, and greater access to reagents helped this molecule and its relatives shift from laboratory curios to strategic scaffolds in modern science.
Product Overview
(4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol embodies a fusion of rigid ring structure and the electronegativity of fluorine, lending it qualities chemists admire. This compound stands out among chromene derivatives for its controlled stereochemistry and dual fluorine substitution, which often improves metabolic stability and bioactivity. As a solid, it usually presents crystalline nature with notable resistance to moisture and air — factors that matter for both storage and handling. Labs rely on its precision in structure for use as a building block, not just for testing biological activity but to fine-tune the properties of the analogs derived from it.
Physical & Chemical Properties
The chromene core, rigid yet flexible enough for chemical play, brings stability alongside reactivity to the table. Those two fluorine atoms, snug at positions five and seven, push electron density and shift the molecule’s reactivity, changing both how it binds in target proteins and stands up to metabolic enzymes. Melting points for compounds in this class run high, often facilitating purification and minimizing unwanted transformation under usual lab conditions. The polarity introduced by the hydroxyl group makes it soluble in polar organic solvents but less so in water, driving choices at every stage from synthesis through to biological testing. Fluorine itself reduces the molecule’s susceptibility to oxidative metabolism, a feature that medicinal chemists see as a plus in the fight for longer-acting drugs.
Technical Specifications & Labeling
Producers supply (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol as a high-purity solid, generally above 98%, reflecting the need for reliability in further chemical transformations. Standard vial packaging, clear batch documentation, and lot-specific certificates of analysis put buyers in shape to trust what they’re receiving. On labels, you spot the IUPAC name, stereochemistry, molecular formula (C9H6F2O2), molecular weight, and handling guidance. Labs that care about trace impurities move quickly through NMR and mass spectrometry readings, checking for isomeric contamination or fluorine migration — rare, but possible with less refined techniques.
Preparation Method
Synthesis draws on ring annulation strategies, typically starting from resorcinol derivatives. Chemists protect sensitive groups, carry out regioselective fluorination with either electrophilic sources or metal-mediated processes, often followed by a reduction process to install the 3,4-dihydro fragment. Success in this chemistry relies on controlling reaction temperature, order of reagent addition, and precise stoichiometry. Most modern workflows avoid toxic intermediates, swapping out harsh reagents in response to environmental pressures and lab safety policies. The chiral center at position four calls for asymmetric catalysis or resolution steps, which, though labor-intensive, lift the product to the level needed for advanced applications.
Chemical Reactions & Modifications
Once in hand, (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol opens itself to reaction at either the hydroxyl site or the aromatic core. Chemists process the alcohol via etherification or esterification to tune solubility and transport properties. Electrophilic aromatic substitution still finds traction on the non-fluorinated rings, while Suzuki and Buchwald-Hartwig couplings extend the structure for more complex targets. Oxidation and reduction reactions lead to rings incorporating new heteroatoms or break the aromaticity for further diversification. In hands-on research settings, transforming this molecule often centers on making targeted derivatives for activity screens, feeding the engine of drug innovation.
Synonyms & Product Names
Depending on catalog, region, or context, you see different tags – from “5,7-Difluorochroman-4-ol” to cryptic identifier codes in chemical supply databases. Researchers cross-reference CAS numbers against supplier lists and frequently encounter nickname shorthands in research articles. Documentation always comes back to the core IUPAC nomenclature, especially for publications and patent filings. The practical upshot of strong naming discipline is transparency in sourcing and safety evaluation.
Safety & Operational Standards
As with most aromatic fluorinated compounds, the toxicity profile for (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol stays lower than that of many industrial fluorochemicals, in part because of the controlled placement of fluorines and the molecule’s modest size. Inhalation, ingestion, and dermal exposure call for gloves, eye protection, and good ventilation at all stages, especially during large-scale manipulations. Chemical hygiene plans in well-run facilities prioritize spill containment, proper waste disposal, and tracking of fluorinated organic residues — habits that serve well beyond regulatory box-ticking. Fire response generally leans on dry chemical powder and CO2, never water sprays due to risk of hydrofluoric acid from incomplete combustion of the organofluorine fragment.
Application Area
Medicinal chemistry stands as the main playground for this chromene, where the marriage of ring rigidity and fluorination promises activity across antitumor, anti-inflammatory, and CNS-related screens. Crop science draws on its stability profile and resistance to enzymatic degradation for use in agrochemical innovation, chasing longer persistence in field trials. Specialist coatings and polymers also look to the basic skeleton for its weather resistance. Analytical chemists, always looking for stable isotopic markers, find value in the predictable NMR shifts of its fluorines. The tapestry of its uses grows as more teams dig into what these tailored structures can bring to problems at the pharmacy, in the field, or even in advanced materials labs.
Research & Development
Many lead optimization teams treat (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol as a privileged scaffold for SAR studies. Its high degree of functionalizability means it lands on screening decks in fragment libraries and advanced molecular modeling runs. Academic groups probe its ability to disrupt protein-ligand interactions by crystal structure work or broad-spectrum binding assays. In pharma and biotech, researchers map metabolism pathways by spiking it into hepatocyte cultures, then trace both stability and breakdown products. Every iteration involves a balance — retaining potency, smoothing out ADME liabilities, and hopefully dodging toxicity flags as they inch closer to a clinical candidate or a novel crop protection product.
Toxicity Research
Animal studies on similar difluorinated chromenes point to lower acute toxicity, especially compared to fully perfluorinated analogues, but vigilance persists over the unknowns of chronic exposure and subtle organ buildup. Enzyme inhibition screens as well as off-target activity in neurological tissues get close review. Environmental impact checks focus on the breakdown in soil microbes and resistance to regular wastewater treatment, a challenge that drives further study into controlled degradation or safer analogs. Laboratories keep current by tracking studies published in toxicology journals, regulatory agency bulletins, and ongoing EU/US risk assessments for organofluorine molecules.
Future Prospects
Interest in these fluorinated chromenes grows every year, as their track record matures across both pharmaceuticals and specialty materials. Some see value in pairing them with other functional groups keyed for targeted delivery or increased bioavailability, using modern catalysis and green chemistry. Demand for eco-friendly manufacturing routes pushes chemists to cut hazardous reagents and shrink solvent footprints, and companies now advertise greener syntheses as a competitive edge. In drug development, ongoing computational design, better screening models, and a move toward individualized medicine all look to adaptable core structures like this one. As regulation of persistent fluorinated chemicals tightens, teams stay alert for both risks and next-generation replacements, aiming not just to meet compliance but to anticipate future needs and opportunities.
Understanding the Molecule Beyond Its Name
The name (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol does not exactly roll off the tongue, but its structure tells a story. This compound, sitting in the chromene family, brings a couple of fluorine atoms to play, which makes it stand out in both pharmaceutical circles and broader chemical research. A molecule like this grabs attention because of what those fluorine substitutions can do to the biological and chemical behaviors typically seen in chromene derivatives.
The Influence in Drug Discovery and Design
Many folks in drug research will recognize the chromene backbone. By adding fluorine, the molecule takes on new life. That fluorine offers more than a chemical tweak—it can slow down how quickly enzymes chop the compound up in the body or help the molecule slip through cellular walls. In practical terms, researchers often turn to this sort of structure when hunting for candidates to fight tumors, inflammation, or microbial threats. A string of papers over the past decade highlights how these compounds, with a difluoro touch, can trip up certain enzymes tied to cancer progression or disrupt bacterial growth.
What makes this even more important: adding fluorine to a structure often brings longer-lasting and more predictable effects inside the body. Big pharmaceutical companies continue to invest piles of money into optimizing natural and synthetic compounds by just swapping in a halogen or two. Even if (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol itself has not yet become a medicine, its chemistry gives scientists a much-needed starting point.
Role in Synthesis and Chemical Method Development
Outside of medicine, chemists often use molecules like this to test new ways to build more complex frameworks. In my own graduate lab days, templates like chromenes served as proving grounds for catalysts and new synthetic routes. By tweaking groups on the ring, such as popping in two fluorine atoms, you do not just end up with a new molecule—you're also learning how various electronic shifts change reactivity. These lessons ripple out into better techniques that can turn up in fields from agrochemical pipelines to new dyes and electronic materials.
Potential in Environmental and Material Science
Some research teams are looking at difluoro-chromene compounds for their stability and resistance to environmental breakdown. If you have ever struggled with finding a compound that won’t degrade in sunlight or break down in water, adding fluorine can help. This makes these molecules promising in the hunt for rugged coatings or advanced plastics. More specific uses depend on tuning the chemistry for each job, but the backbone offers a jumping-off point rich in possibility.
Moving Forward with Purpose
One problem keeps coming up: the balance between innovation and long-term safety. Adding fluorine may give rise to better, longer-lasting compounds, but it also raises questions about persistence in the environment. We have already seen concerns over PFAS and related compounds. For each win in durability, chemists have to run thorough safety checks and push for greener, more breakable versions where possible.
Scientists, companies, and regulators work together on solutions that meet real needs without risking future harm. The next few years will likely see this molecule—or something close to it—pop up in journals and labs, not just for what it can do, but for the lessons it offers about making better choices in chemistry.
Digging Deeper Than the Label
Many products claim a certain chemical purity or grade. These labels help guide decisions in labs, factories, and even classrooms. From my experience in research settings, relying only on what's mentioned on packaging can lead to missteps, wasted money, or worse—flawed results. Those labels—“ACS Reagent,” “Laboratory Grade,” “Technical Grade,” “USP,” and so on—offer a snapshot, but the real story sits in the details.
Numbers That Speak Volumes
Purity gets measured by percentage—for example, 99.9% pure sodium chloride. That single number doesn’t capture the impact trace impurities can carry. While 99.9% looks impressive, the 0.1% leftover can matter a lot. In electronics or pharmaceuticals, minuscule contamination disrupts the process or makes products unsafe. I've seen research projects derailed by a supplier swapping their reagent grade chemical for a technical grade alternative, thinking, “It's close enough.” The experiments failed, and the cleanup cost more than choosing the right grade at the start.
Regulations, Safety, and Trust
Labels like “USP” and “Pharmaceutical Grade” do more than advertise quality. The U.S. Pharmacopeia (USP) sets rigorous standards demanded by law for medicine ingredients in the United States. Companies risk lawsuits—or worse—if their products contain off-spec contaminants. In 2022, an FDA recall targeted lots of magnesium citrate because levels of microbial contamination slipped past controls. Lives and company reputations faced real harm. High grade isn’t just for show; it’s a legal, ethical, and safety requirement.
Grades—Much More Than Marketing
Lab supply catalogs love their terms: ACS, Technical, Food Grade. Each signals different impurity limits and intended uses. Science teachers might get by with lab-grade chemicals for basic demonstrations, but researchers working on cancer drugs or computer chips demand tighter controls. “Food grade” carries expectations of non-toxicity even at low doses, while “technical grade” favors bulk processes—think fertilizers or industrial cleaners—where small impurities don’t ruin the process or pose a health risk. Nobody wants a contaminated breakfast cereal or a failing battery in their car because the wrong grade slid through.
Solving the Purity Puzzle
Scrutinizing Certificates of Analysis (COA) should become a habit, not an afterthought. Reputable suppliers make these accessible. The COA reveals specifics—including trace metals, moisture, or even residual solvents—that help buyers spot risks and ensure compliance. Cross-checking supplier data against recognized standards, such as ISO 9001 or national pharmacopeia, adds another layer of security. Trust comes by building relationships with reliable suppliers; reputable companies rarely cut corners since their reputation depends on it.
Education: The Quiet Key
From my own work, team members make fewer mistakes when they learn to ask pointed questions: What is the minimum acceptable grade? What will trace levels of x impurity change in our process? Education keeps people safe and projects successful. Public databases, regulatory guidance, and transparency from suppliers all help. In the end, understanding chemical grades and purity means protecting health, science, and business interests—none of which deserve shortcuts.
Protecting What Matters: Chemical Stability Depends on How You Store It
Many folks working with lab chemicals know that stability can make or break your experiment, especially with tricky molecules like (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol. Cutting corners with storage means you waste time and resources. Over the years in chemical labs, I’ve seen researchers lose entire weeks of work just because a sensitive reagent broke down. It’s not always about temperature or darkness—humidity, air, and even the material of the storage bottle play a role.
Temperature: Slow Down Degradation
It’s tempting to leave everything on a shelf at room temperature, but organic molecules like this one often react with air or light. The literature and experience both say that cold storage helps curb those unwanted side reactions. I always reach for the -20°C freezer whenever there’s a chiral center or a fluorinated aromatic ring in the mix—(4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol has both. There’s evidence from small-molecule stability studies showing higher retention and repeatable spectroscopy results when molecules like this stay cold.
Avoiding Light: You Can’t Trust Fluorinated Chromenes with UV Exposure
Leaving a white powder under a fluorescent light for a few days might not look dangerous, but photo-degradation creeps up. Many chromene and coumarin analogs react to UV rays in ways invisible to the naked eye. I’ve seen sample color shifts and faint odors develop from what should have been a stable powder. Storing inside amber glass vials keeps damaging rays out. Some researchers even wrap vials in foil for an extra layer, and I’ve adopted this habit after seeing the cost of failed samples stack up.
Air, Moisture, and Contamination: The Enemies of Purity
I’ve spilled too many samples because I used the wrong bottle stopper or didn’t check for desiccant. Any exposure to humid air or oxygen invites hydrolysis or oxidation. These reactions shoot impurities through the roof, especially with molecules containing hydroxyl groups. Lab practice—and various chemical safety databases—recommend using tightly sealed containers with a desiccant packet inside. Plastic containers tend to leach or let in trace vapors, so I stick with glass. Even better, flushing the vial with a burst of nitrogen before sealing keeps oxygen out.
Clean Handling: Every Little Bit Counts
Nothing ruins your day like starting with a pure powder and ending up with a contaminated mess. Good storage starts with clean hands, clean spatulas, and dedicated glassware. Labeling everything with the date makes it possible to spot problems before they spiral. Insiders learn that even the type of tape matters: masking tape can leach glue vapors into a vial! A reliable labeling pen, crisp lab tape, and a clear record keep surprises out of sensitive chemistry.
Solutions Worth Considering
Spend the extra few minutes to store (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol in a dry, airtight amber glass vial inside a -20°C freezer. Add a silica gel pack, flush with nitrogen, and label each vial well. This effort goes a long way toward preserving chemical purity and making sure those research hours count. Labs that set up a simple protocol for storage see fewer lost samples and save money, all while building better habits for handling every new compound down the line.
A Closer Look at Safety Practices
Picking up a bottle of any chemical, it’s tempting to just glance at the label and call it good. For folks working in labs, factories, or even universities, that shortcut can kick off a chain of headaches—sometimes with serious consequences. Each compound comes with its own hazards. Acids burn skin, solvents give off dangerous fumes, and some powders linger in the air, just waiting for someone to breathe in a dose.
Spending a few years in a lab, I saw how routine makes it easy to overlook basics. Gloves start to feel optional, goggles fog up and get set aside, and pipettes get used for something they shouldn’t. These moments give small mistakes a chance to become accidents. Focusing on safety isn’t just a lecture point from a safety officer. The stories behind each incident drive the point home: a forgotten label led to acid burns; someone skipped gloves and broke out in a rash. Real people, real setbacks, and sometimes real, lasting harm.
Labeling and Storage—Simple Steps with Big Payoffs
Every step, from receiving a new shipment to moving something between benches, calls for careful attention to labeling. Different chemicals can look pretty similar in clear bottles or flasks. Labels with the full chemical name, hazard class, and handling instructions cut confusion. Safe storage matters just as much. Acids and bases don’t belong together, and strong oxidizers need space away from things that burn. Many of the worst spills and accidents I saw came from rushed storage—stick acids with the acids, even if it takes a little longer to sort out the shelves.
I’ve seen what can happen when containers get left open. Solvents evaporate, raising health risks and fire hazards. Simple habits make all the difference: keep lids tight, note the opening date, and rotate stock.
Understanding the Data, Not Just Collecting It
Material Safety Data Sheets (MSDS) or Safety Data Sheets (SDS) aren’t just paperwork for compliance. They spell out the health hazards, first aid instructions, and steps to take if a spill happens. Reading that document before working with something new turned into a habit for me, because learning about the risks during a spill or exposure is the worst way to do it. The warnings about inhalation, skin contact, or eye exposure aren’t suggestions—they’re the voice of years of collected cases and injuries speaking up for your safety.
Personal Protection Isn’t Optional
Some people say breathing masks or goggles feel uncomfortable or overkill. In practice, a few extra minutes setting up personal protection beats days out of work with a chemical burn or lung problems. Nitrile gloves handle most routine tasks, but some compounds need better coverage. Splash goggles help out when pipetting acids or handling anything that might splash. Respirators come into play for dusty or volatile chemicals. I watched seasoned chemists skip these steps once or twice, and the risks caught up to them in sneaky ways—a drop in the eye, a cough that didn’t leave after a shift.
Training and Paying Attention
Many organizations run annual or even monthly training on handling and safety. Some use horror stories to drive the point home, others stick to the basics. The best teachers walked through real outcomes from local labs—an incident, the steps missed, and what would have changed things. Practicing spill clean-ups, using fire blankets, or doing eye wash drills makes it easier to act decisively under real stress.
Dealing with chemicals safely strips down to common sense, taking your time, and refusing to cut corners. A strong safety culture doesn’t just protect hands and lungs—it keeps projects moving, reputations strong, and teams healthy enough to do work they care about.
Why NMR and MS Data Matter for Chemists
As someone who’s spent late nights over a spinning NMR tube, analytical data feels less like optional details and more like the foundation that holds any organic project together. For a molecule like (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol, every fluorine atom and every chiral center drives up the stakes for unambiguous characterization. Without clear Nuclear Magnetic Resonance (NMR) or Mass Spectrometry (MS) profiles, trust in structure or purity flies out the window—whether you’re working in pharmaceuticals, flavor chemistry, or academic research.
Struggles Finding the Right Data
Digging through common databases such as SciFinder, PubChem, and Reaxys turns up plenty of technical details but not always the raw spectra for niche compounds. If you punch in “(4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol,” a flurry of similar chromenols and fluorinated scaffolds might pop up, but direct matches for full NMR or high-resolution MS data? More often than not, these remain elusive or paywalled in journal supplements. That’s a real barrier for lab teams or smaller startups working with tight budgets who can’t shell out for every article behind a paywall.
Why Some Compounds Are Hard to Source Data For
Deals with journals and chemical vendors draw a hard line between public information and proprietary data. Sometimes, research into analogs of chromen-4-ol structures gets wrapped up in patents, or the compound doesn’t make it into a publication at all. If a molecule hasn’t gone through the academic peer-review process or a vendor hasn’t made it a catalog staple, the data stays locked behind private lab notebooks. In my postdoc years, squinting at hand-drawn spectra felt like a rite of passage—frustrating but essential since not every unusual molecule comes with a published set of spectra.
Gaps Create Risks and Slow Progress
Any chemist chasing a synthetic route knows the routine: you need to know the NMR splitting patterns, the MS fragmentation, and those little quirks that show whether you’ve nailed the right stereochemistry. Missing or limited spectral data means researchers spend extra hours running their own analyses, sometimes duplicating work that’s already finished elsewhere. This leads to inefficiencies across the board, especially for novel drug leads. In some cases, the absence of robust, published data lets spurious results slip through—in one infamous synthesis paper, questionable NMR assignments weren’t caught for months, delaying everything from grant applications to graduate theses.
Pushing for More Transparency and Data Sharing
We can break out of these silos. Standardized repositories—like the NMRShiftDB or the MassBank—offer glimpses of what’s possible when raw spectra are made widely available, not tucked away behind subscriptions. Open journals have started requiring spectra as supplementary info, a good move that should become normal practice. Community initiatives, such as ChemSpider and collaborative annotation projects, also help surface data. Synthesizing rare analogs? Upload those NMRs somewhere public. Startups can speed their own research by sharing anonymized data—protect the project but help the broader community.
Better Access Means Safer and Faster Chemistry
Chemistry thrives on verification. By building a stronger culture of open data—especially for complex, multi-substituted compounds like (4R)-5,7-Difluoro-3,4-dihydro-2H-chromen-4-ol—everyone wins. Labs become more effective, students learn real spectra not just textbook patterns, and scientific integrity improves without extra cost or complexity. The future of research looks brighter with open doors—and more annotated FIDs and m/z plots just a quick search away.