4-Fluoronitrobenzene: A Closer Look at an Industrial Chemical
Historical Development
4-Fluoronitrobenzene came onto the scene during a period when chemists drove industrial progress with new aromatic compounds. Years after the first nitrobenzenes changed dye manufacture and explosives, scientists in the mid-twentieth century saw promise in halogenated aromatics, including this specific fluorinated nitrobenzene. Lab records point to its arrival around the time that researchers understood the power of placing electron-withdrawing groups on an aromatic ring. This compound took off in pharmaceutical and agrochemical circles, too, since its structure made it a useful intermediate for synthesizing more complex chemicals. By the late 1900s, large-scale chemical facilities in Europe and the U.S. ran streamlined processes to meet surging demand from researchers and industry.
Product Overview
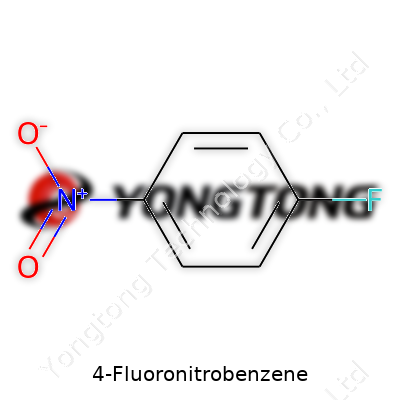
4-Fluoronitrobenzene holds a niche in organic chemistry as both a reactive building block and an end-use chemical in its own right. Its molecular formula, C6H4FNO2, tells a story of structure-based utility: the para-position fluoro group guides reactivity, and the nitro group enables reduction and substitution reactions key to so many downstream compounds. Labs see it as an accessible starting point for engineering more valuable molecules, so it stands at the center of processes making medicines, crop protectants, and electronic materials.
Physical & Chemical Properties
This aromatic chemical forms pale yellow crystals or sometimes oils when stored above room temperature. It melts at roughly 50-55°C and boils around 215°C. Solubility in water remains quite low, but it dissolves well in typical organic solvents such as ethanol, acetone, and ether. With a molecular weight near 141 grams per mole, 4-fluoronitrobenzene signals its reactivity through both the nitro and fluorine atoms, both of which change how it interacts with nucleophiles and electrophiles in practical synthesis. Its density (about 1.37 g/cm³) and vapor pressure matter for storage and transport, reminding handlers that even seemingly stable compounds need oversight against leaks or spills.
Technical Specifications & Labeling
Any shipment or stockroom bottle of 4-fluoronitrobenzene comes labeled with purity grades, often better than 98% for technical or synthetic use. Chemical suppliers include CAS number 350-46-9 and carry hazard pictograms as required under GHS standards, flagging both acute toxicity and environmental risk. Labels spell out “4-fluoronitrobenzene” and common synonyms, so even non-specialists quickly recognize hazards or can cross-check inventories. Barcodes and batch records track lot-to-lot variation, which matters especially where strict specifications come into play, as in pharmaceutical synthesis.
Preparation Method
Industrial routes for making 4-fluoronitrobenzene typically start with fluorobenzene. Controlled nitration, using a mix of concentrated sulfuric and nitric acids, produces a mixture of isomers. The para-isomer dominates under conditions where temperature and acid ratios slow down the reaction, since steric effects steer the incoming nitro group accordingly. Some setups go a step further, purifying through distillation or recrystallization, to get a single product suitable for sensitive downstream reactions. On smaller scales, lab workers work in fume hoods, since evolving vapors and heat make this process anything but routine.
Chemical Reactions & Modifications
Few compounds serve as such flexible intermediates. The electron-deficient ring of 4-fluoronitrobenzene invites nucleophilic aromatic substitution, allowing scientists to swap the fluorine atom for amines, thiols, or alkoxides. The nitro group converts to an amine with reduction methods, forming 4-fluoroaniline, another high-value molecule in dye and pharmaceutical chemistry. In my own work, I’ve seen it used to prepare complex heterocycles—by combining this building block with an amine in a sealed tube, the reaction only needs heat and time to yield new classes of ligands or medicinal precursors. Its mild volatility reminds chemists to harness its reactivity with care.
Synonyms & Product Names
Researchers and suppliers refer to this chemical both as 4-fluoronitrobenzene and p-fluoronitrobenzene. Older catalogs also list it as 1-fluoro-4-nitrobenzene and para-fluoronitrobenzene. I’ve come across abbreviations such as 4-FNB or simply FNB in laboratory records, but full naming routines remain a good habit for clarity. Chemists keep tabs on registration numbers for import and export, given that similar isomers show up in various regulatory databases.
Safety & Operational Standards
Working with 4-fluoronitrobenzene means following strict rules in the lab and in industry. It poses moderate acute toxicity by both inhalation and skin contact, so gloves, splash goggles, and rotating the use of lab coats stay standard for all staff. Ventilated fume hoods run when transferring or reacting the compound since inhaling vapors risks respiratory irritation or longer-term effects. Training sessions for handling nitroaromatics highlight spill control, first aid guidelines, and storage under tight-sealing containers. Facilities include fire extinguishers suitable for organic compounds and keep safety data sheets within arm’s reach of the workspace. Regular drills and compliance with local chemical handling regulations keep users and the environment safer.
Application Area
Industries from pharmaceuticals to crop sciences call on 4-fluoronitrobenzene for its flexibility. Its main use remains as a synthetic intermediate, bridging the gap between simple aromatics and more valuable complex molecules. Factories and research labs make specialty dyes, modern agrochemicals, and some advanced materials using it as a building block. In my own years working with chemical startups, chemists valued its reactivity to (re)design molecules for improved biological activity or better stability. Over the last decade, electronics-focused firms started to pay attention too, since fluorinated aromatics often bring new properties to organic semiconductors. Clearly, the push for novel technologies boosts demand and shapes future research.
Research & Development
Labs worldwide invest significant effort into refining how 4-fluoronitrobenzene gets made and what it can become. Ongoing research tries to improve selectivity in the nitration of fluorobenzene, aiming to reduce waste and save both money and energy. Green chemistry ideas get traction: milder acids, alternate solvents, and possibly even enzymatic routes to aromatic nitration. Medicinal chemists see it as a cornerstone for rapid library synthesis, offering opportunities to discover new leads for treating infectious disease or cancer. Cross-disciplinary teams at universities examine how fluorine placement affects drug metabolism or pesticide breakdown—a step that depends on careful recording of reactions and outcomes.
Toxicity Research
Safety officers and toxicologists took an early interest in the health risks linked to 4-fluoronitrobenzene. Animal studies show that high doses cause irritation to mucous membranes and the skin. Oral or inhaled doses above regulated limits trigger methemoglobinemia, a blood disorder that affects oxygen transport. Chronic exposure raises concerns around organ toxicity, though well-managed workplaces keep these risks low. Guidelines from occupational health authorities recommend regular monitoring of air concentrations and strong personal protective equipment. Waste streams pass through treatment facilities designed to neutralize nitro compounds before release, as both worker safety and public environmental health demand strict controls.
Future Prospects
Looking ahead, 4-fluoronitrobenzene stands to remain relevant as both a trusted building block and an example of responsible chemical handling. Molecular innovation across pharmaceutical, agricultural, and materials fields drives ongoing demand. Scientists hope to find cleaner synthetic routes, perhaps tapping into electrosynthesis or new catalysts that slash waste and energy use. Regulatory agencies worldwide will almost certainly tighten oversight on nitroaromatics, so tracking every shipment, disposal, and exposure event stays front of mind. Advances in green chemistry promise to change how we view aromatic halonitro compounds, tipping the balance toward safer, more sustainable chemical industries. That progress only happens with a steady stream of research, active sharing of safety data, and new policies that track with scientific understanding.
Uses Rooted in Real-World Needs
Chemists reach for 4-Fluoronitrobenzene in labs and factories for a simple reason: this compound has the right qualities for building more complex and valuable chemicals. I’ve seen its reputation grow especially in pharmaceutical and crop protection work. That’s not about hype, but about what this compound can actually do as a building block. It offers up its nitro and fluoro parts, letting scientists swap these out or tweak them to change how new chemicals behave.
Pharmaceutical Production: Essential Steps
Working in a lab, you often juggle a series of reactions to create the molecules that will end up in pills or injectables. 4-Fluoronitrobenzene becomes one of those essential steps, especially for drugs containing aromatic rings decorated with tricky substituents. That fluorine atom changes biological activity—sometimes it blocks metabolic breakdown or helps a medicine reach a target in the body. The nitro group gives chemists more options, too. It can be reduced to an amine, for instance, opening paths toward a dozen new molecules. There’s a smooth logic behind why this compound shows up so often in patents: it helps unlock potent antiviral, anti-inflammatory, and even cancer-fighting drug structures.
Role in Crop Protection Chemicals
I’ve talked to folks in agriculture who count on chemical innovation to keep farms productive. Here again, 4-Fluoronitrobenzene plays a part. Its structure makes it useful in building up herbicides and fungicides with selective toxicity—compounds that fight off pests and mildew but don’t mess up the crops. The fluoro and nitro groups sharpen the target and sometimes let smaller doses do the job, which helps both farmers and the environment. That’s something anyone who eats or grows food can appreciate.
Manufacturing Challenges and Safety
There’s no getting around the fact that 4-Fluoronitrobenzene requires careful handling. Working with nitro aromatic compounds brings risks—fire, explosion, and health hazards if proper safeguards are ignored. The industry relies on trained people, strong ventilation, and gear that keeps contamination and accidents at bay. There’s also a regulatory side; those making or selling it must track usage, ensure safe shipping, and follow rules that keep workers and the public safe. I’ve seen how this responsibility can slow down projects, but it matters more than speed ever will.
Seeking Safer and Greener Alternatives
Many researchers wonder if next-generation chemistry could drop some of these hazards. The demand for greener processes keeps growing, mostly because communities want less pollution and scientists want less personal risk. Methods like catalytic hydrogenation, safer solvents, and “flow chemistry” setups all point the way forward. There’s a movement to design building blocks with fewer environmental drawbacks, but changing over takes time and money. Still, as long as the markets for innovative medicines and crop protectants keep growing, it makes sense to push for smarter ways to make and use compounds like 4-Fluoronitrobenzene.
What Is 4-Fluoronitrobenzene?
Picture a benzene ring—six carbon atoms linked in a sturdy loop, hugging each other with bonds that don’t easily budge. Now, add two distinct guests to the party. Sitting at the "4-" spot, a fluorine atom swaps in for a hydrogen. Across the ring at the "1-" position, a nitro group takes its place. That’s 4-Fluoronitrobenzene in a nutshell.
Chemical Formula: C6H4FNO2
Four hydrogens attach to the benzene core, sharing space with a single fluorine and a single nitro group (NO2). The rest of the ring keeps its familiar carbon backbone. It looks simple written out, but the effects of substituting one hydrogen for a fluorine and another for a nitro group go far beyond what a formula shows on paper.
Why 4-Fluoronitrobenzene Matters in Labs and Factories
This molecule isn’t just lab wallpaper. Chemical industries turn to it when designing dyes, pharmaceuticals, and pesticides. Fluorine’s presence tweaks its chemical attitude. For chemists, that means they get to play with reactivity on their terms, not the molecule’s whim. The nitro group brings different traits: at once electron-hungry and eager to react further. With both groups on the ring, you get a compound that serves as a crucial building block in industrial syntheses.
Producing 4-Fluoronitrobenzene tends to rely on nitrating fluorobenzene, which itself takes serious care to manage—think strong acids, controlled temperatures, tight safety rules. Having been around various university and industry chemical storerooms, the protocols never feel like overkill. This stuff doesn’t take mistakes lightly. Even a small spill or improper storage turns a routine day into a scramble, with safety showers and fume hoods suddenly earning their keep.
The Health and Environmental Picture
No one tosses this compound around casually. Breathing in its dust, getting it on skin, or failing to properly contain it can backfire, leading to irritation or worse. Waste and byproduct disposal carries its own headaches—nitro-compounds don’t break down easily, so they stick around and mess with local water and soil. That’s a real issue for communities living near manufacturing sites. Having grown up near a plant that handled chemical intermediates, I can recall mornings with odd smells in the air, and warnings from local news about runoff. Chemicals like these may seem distant to most people, but the risks demand respect.
What Can Be Done Better?
Better safety training tops the list. Companies need to keep workers sharp and aware, not just when onboarding, but every year. Investing in top-tier ventilation and spill containment keeps trouble from spreading. The industry also owes it to local neighborhoods to upgrade waste treatment—advanced processes, like biodegradation using engineered bacteria, have started catching on. Regulatory oversight shouldn’t rest on self-reporting and paper trails. Surprise inspections and transparent communication between plants and residents build vital trust.
It’s tempting to focus only on formulas and lab work, but 4-Fluoronitrobenzene shows how a single chemical sits at the intersection of industry, community, and environment. Producing and using it responsibly means bridging the gap between science and daily life.
A Closer Look at 4-Fluoronitrobenzene
4-Fluoronitrobenzene comes up often in the world of chemical manufacturing, especially for products like pharmaceuticals or dyes. It packs a nitro group and a fluorine atom onto a benzene ring—both changes that can turn an otherwise common molecule into something concerning if handled wrong. Many in the lab world have seen its yellow, crystalline form and know the warning labels are there for a reason.
Health Risks Connected to 4-Fluoronitrobenzene
Touching or breathing in this compound brings risks that aren’t just hypothetical. The nitro group in particular makes the molecule reactive and able to cause harm in a hurry. If powder gets on your skin, expect redness, irritation, and maybe lasting dryness. Getting it in your eyes raises stakes to burning pain and possible damage. The real worry starts with inhalation or swallowing. Such exposure can lead to headaches, nausea, dizziness, and potentially bigger troubles for the kidneys or liver over time. Incidents from chemical plants show careless handling can end with hospital trips.
Long-term or repeat exposure isn’t just about shortness of breath or feeling unwell. Professional guidelines flag 4-Fluoronitrobenzene for its suspected ability to impair organ systems—not a chemical to take lightly. Looking at animal studies and workplace reports, symptoms echo the classic signs of toxic nitrobenzene exposure: blue-tinged skin, confusion, and even loss of consciousness as oxygen carrying gets disrupted in the blood.
Environmental Considerations
Chemicals like 4-Fluoronitrobenzene don’t just disappear after they’re used. Water, air, and soil can all carry residues from spills or improper disposal. The fluorine and nitro parts resist breaking down, sticking around long after waste leaves the factory. Local wildlife can suffer, especially fish and insects living in streams near disposal sites. Long-range transport in water can spread the issue far past the original spill.
Regulatory agencies like the EPA and European ECHA treat fluorinated nitro compounds as substances of concern. Factories must report usage and spills. The need for careful containment and dedicated waste processing isn’t just red tape—it’s based on real ecological damage observed in cases of chemical runoff.
Protecting People and Places
Working safely with 4-Fluoronitrobenzene always means personal protective equipment: gloves, goggles, lab coats, and sometimes respirators. Even a quick transfer or spill demands fast cleanup and well-ventilated spaces. Training helps—people who understand the risks seldom skip the precautions.
Disposal stands as a sticking point in the industry. Trusted approaches include incineration at high heat and specialized chemical neutralization, never pouring waste down the drain. Regulatory oversight pushes firms to track volumes and maintain full safety logs. A company can’t afford to cut corners with a molecule like this, especially as new laws get drafted.
Room for Better Solutions
Chemical safety systems improve every year, but lessons from older incidents show why constant vigilance matters. Substitution remains a priority in research and industry: swapping 4-Fluoronitrobenzene for less hazardous options wherever possible. Newer synthetic methods sometimes allow this swap without a loss in product quality. For now, respect for facts—backed by research, industry experience, and government rules—shapes the safest path forward.
Simple Chemistry, Real Dangers
Walking through a chemical storeroom, you notice right away that some bottles carry warnings you don’t want to ignore. 4-Fluoronitrobenzene deserves your respect in any lab or industrial setting. It's not just another bottle on the shelf, because its properties include volatility, toxicity, and the potential for hazardous reactions.
The Risks Are Not Theoretical
Anyone who has ever worked with nitro compounds quickly learns you don’t want fumes building up indoors. Vapors may irritate the nose and throat with little warning. Spills on the skin sting, so direct handling without gloves is out of the question. 4-Fluoronitrobenzene falls in line with these issues, plus it reacts with strong reducers or bases. Fires aren’t common, but in the right scenario, sparks, heat, or incompatible storage can set off an emergency. Don’t forget the long-term health effects, either—chronic exposure concerns keep chemical safety officers awake at night. If regulations follow REACH or OSHA guidelines, those aren’t just boxes to tick; enforcement happens for good reason.
Best Storage Practices
To keep things safe and compliant, I always favor small, well-marked containers made of glass or chemical-resistant plastic. Tightly sealed lids stop fumes from escaping into the storeroom air. Labeling makes a difference—use clear, durable tags that last through cleaning chemicals, just in case. Anyone reaching for them later should know what’s inside at a glance. Shelves keep containers upright, out of reach from vibrations and away from potential spills.
Chemical compatibility charts aren’t just wall art; they save more than paperwork headaches. 4-Fluoronitrobenzene stands apart from oxidizers, acids, strong bases, and anything with a tendency to catch fire. That means separate cabinets labeled for organics, and definitely no stacking next to bleach, ammonia, or peroxides. Metal shelves risk corrosion, so go for painted, coated, or polyethylene-lined surfaces.
Temperature makes or breaks chemical safety. Keep storage cool, dry, and out of direct light to slow down unwanted reactions. Forgetting this can accelerate chemical breakdown, rupture containers, and spill toxic residue across a storeroom floor. Most labs keep rooms at less than 25°C (77°F), and air conditioning isn't just for comfort—it’s your defense against problems you might not smell or see right away.
A Shared Responsibility
Experience shows that safety routines save time and money in the long run. I’ve seen teams scramble during audits because training skipped routine checks. The best labs run regular inventory, inspect for leaks, and remove old stock before it turns into waste disposal headaches. Think of eye wash stations, chemical spill kits, and MSDS sheets posted nearby—these prepare everyone for quick action, not just compliance. If the storeroom feels organized, that’s a direct reflection of a safety-first mindset.
Moving Beyond Storage—Solutions Worth Sharing
Digital inventory systems help track expiration dates and flag unsafe storage combos. A locked cabinet with limited key access keeps only trained hands near sensitive chemicals. Refresher workshops make safety habits stick, especially with new team members. Sharing lessons from minor incidents—what nearly went wrong and why—helps turn small mistakes into useful learning moments. Staying updated with current guidelines from trusted sources like the CDC, EPA, and regional workplace regulations keeps storage practices grounded in science and experience.
Why Purity Matters So Much
Anyone who’s tried to work with chemicals in a lab understands that quality can make or break an experiment. Purity isn’t a background detail, just ask the synthetic chemists hauling through a multi-step reaction they’ve repeated for the fifth time because contaminants keep messing with their yields. Get a batch of 4-Fluoronitrobenzene that’s promised at ≥99% by HPLC and you’ll breathe a little easier. No anxiety about stray peaks showing up and forcing you back to square one. That’s not just time lost, but wasted materials, resources, energy, and—honestly—good moods too.
Understanding the Numbers
Lots of suppliers advertise the 99% mark. The reality is many serious customers—drug discovery folks, research chemists, those responsible for regulatory filings—look for even clearer details. Besides HPLC purity, analysts often want to see GC and NMR traces, melting points, and maybe a detailed certificate of analysis (CoA). For any 4-Fluoronitrobenzene to qualify as “research grade,” it really does need to display minimal organic solvent residues (such as methanol or dichloromethane), heavy metals, and water content.
Impurities: The Unwelcome Guests
In my own time doing bench chemistry, there was nothing more frustrating than discovering after the fact that a small unknown impurity led to months of confusion. In 4-Fluoronitrobenzene, common pests include other substituted nitrobenzenes, leftover starting materials, and trace acid residues. These might not jump out on the standard TLC plate but show up later, creating headaches as downstream reactions throw up mystery by-products.
Setting Standards: What the Data Shows
Looking through published literature and supplier datasheets, specification ranges for high-quality 4-Fluoronitrobenzene often demand at least 99% purity (by HPLC or GC), colorless-to-pale yellow crystals or liquid, water content less than 0.2% (Karl Fischer titration), and heavy metals below 10ppm. IR and NMR spectroscopies are routine checks too, since a clean aromatic profile on the NMR says a lot about the product’s story. Data transparency in chemical catalogs has improved because buyers demand to see that paperwork before writing a single purchase order.
Solutions and Progress
Across the industry, sourcing departments now vet suppliers more harshly than before—not simply poking around for the cheapest source. Many labs keep a small network of trusted vendors and avoid switching because repeatability means everything. If a supplier batch doesn’t match specification sheets (say, melting point off by a degree or hidden traces of an unknown peak in their chromatogram), researchers often tell their peers, or even blacklist that supplier internally.
For manufacturers, ramping up purification processes (like precision distillation, improved crystallization, or investing in better column purification) pays dividends. On the lab side, smart storage also means less worry over shelf degradation—using argon atmosphere or sealed ampoules to slow down hydrolysis. Techniques aren’t stagnant: regular re-checking of stock samples and running impurities against reference standards now make up day-to-day best practice, especially for anyone prepping large-scale runs.
Small Details Carry Weight
Purity in 4-Fluoronitrobenzene isn’t just a technical issue—it determines if a reaction sequence succeeds, whether analytical methods work smoothly, and whether downstream applications stay safe. That’s something every chemical worker discovers, one batch at a time.