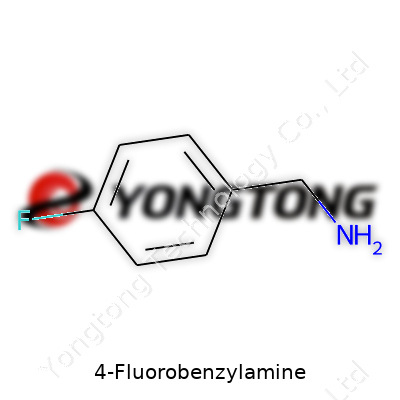
4-Fluorobenzylamine: A Comprehensive Commentary
Historical Development
Chemists first produced benzylamines by modifying aromatic compounds, opening a door for specialized derivatives. 4-Fluorobenzylamine appeared in scientific records by the 20th century, thanks to new methods for introducing fluorine atoms into benzene rings. Laboratories in both pharmaceuticals and agrochemicals started looking to fluorinated amines for their unique electronic properties, and in that rush for molecules with selectivity and metabolic resistance, 4-Fluorobenzylamine gained value. This compound’s story parallels wider advances in synthetic organic chemistry, where specific substitutions on the benzene ring gave rise to new bioactive entities and intermediates for further development. Extensive referencing and academic discussions in chemistry journals trace out how its direct amination and selective halogenation routes matured from rare, costly processes to reliable procedures supporting multi-kilo-scale batches.
Product Overview
This substance has gained notice in the toolkit of research chemists, not only for the unique –NH2 function but mainly because the para-fluorine confers changes in how it reacts in further syntheses. 4-Fluorobenzylamine finds uses as an intermediate for building larger, often more complex, molecules in pharmaceutical research. Drug discovery researchers value how it handles both nucleophilic attack and enzymatic breakdown. Companies obtain it in its standard liquid or crystalline form, packaged in containers that protect against air and moisture to prevent breakdown or contamination.
Physical & Chemical Properties
People working in the lab recognize 4-Fluorobenzylamine by its clear to pale yellow appearance, mild amine odor, and liquid state at room temperature. Typically, it has a melting point around -14°C and a boiling point in the 190-195°C range. Chemists studying this compound note its water solubility, although organic solvents like ethanol or acetone suit it better for most reactions. The amine is quite basic; its lone pair on nitrogen sits directly next to the electron-withdrawing fluorine, tweaking reactivity compared to unsubstituted analogs. This substitution pattern governs not just how it looks or dissolves, but also how enzymes and reagents interact at the molecular level in living systems or reaction vessels.
Technical Specifications & Labeling
Many suppliers provide detailed analytical data before sending the product. The label typically lists the CAS number, molecular weight (125.14 g/mol), and purities that must surpass 98% for research work. Analytical data sheets may feature NMR and IR spectra, as well as precise GC or HPLC tracings to reassure chemists they’re handling the intended compound. Packaging also warns about hazards, including skin sensitivity or toxicity upon inhalation or ingestion. Some containers come in amber bottles, shielded from light, and with tamper-proof seals that indicate product integrity.
Preparation Method
Industrial and academic labs synthesize 4-Fluorobenzylamine through reductive amination or nucleophilic substitution. One popular approach starts with 4-fluorobenzaldehyde, which reacts with ammonia or an alkylated amine in the presence of a reducing agent like hydrogen with a palladium catalyst. Another route begins from 4-fluorotoluene, introducing an amine group using chloramine or other aminating reagents in a stepwise process. Process chemists stress careful control of temperature and pH since side reactions, like dimerization, can crop up. Purification typically uses distillation or recrystallization depending on desired purity, with advanced setups integrating in-line monitoring to minimize waste.
Chemical Reactions & Modifications
Once synthesized, 4-Fluorobenzylamine serves as a launching point for further chemical transformations. Its amine group couples readily with carboxylic acids, leading to amides via standard peptide coupling reagents. To form Schiff bases or imines, chemists react it with aldehydes. For larger-scale, stepwise synthesis in drug or materials science, the para-fluorine keeps the aromatic ring electronically unique; it resists electrophilic attack better than unfluorinated analogs, meaning substitutions occur at predictable positions. Sulfonylation, acylation, or even direct N-alkylation expand the portfolio of derivatives available to medicinal chemists or agrochemical researchers.
Synonyms & Product Names
In chemical catalogs, researchers will find 4-Fluorobenzylamine under synonyms like para-fluorobenzylamine, p-fluorobenzylamine, and 1-(4-fluorophenyl)methanamine. Some inventory systems reference its CAS number, 446-23-9, while others use systematic names such as (4-fluorophenyl)methanamine. Being exact with naming becomes essential, as similar-sounding compounds have very different properties or uses.
Safety & Operational Standards
Lab personnel prioritize gloves, lab coats, and eye protection when handling 4-Fluorobenzylamine. Spills can irritate eyes and skin, and inhalation of vapors leads to respiratory discomfort. Storage usually involves cool, dry places well away from acid chlorides or oxidizing agents. Disposal relies on adherence to local hazardous chemical protocols, which often include incineration or chemical neutralization. Manufacturers provide Material Safety Data Sheets that feature not just immediate concerns but protocols for fire or accidental exposure, so everyone in the lab knows steps to mitigate personal or environmental risk.
Application Area
The biggest demand comes from medicinal chemistry. Pharmaceutical teams leverage 4-Fluorobenzylamine while scouting for new active ingredients, as its fluorine makes derived amides or secondary amines less prone to metabolic breakdown by enzymes. This stability may extend a drug’s presence in the bloodstream or help evade rapid clearance. Agrochemical development uses 4-Fluorobenzylamine to build up selective pesticides or herbicides that benefit from tailored activity profiles or lower toxicity toward beneficial organisms. Material scientists introduce it as a building block for surface modifiers or special polymers, counting on the aromatic ring and fluorine substitution to tweak physical and electronic characteristics.
Research & Development
Research groups dive into structure-activity relationships when they build drug candidates around this amine. Journals often publish results showing that para-fluorination affects not only target binding but also resistance to degradation by monoamine oxidases or cytochrome P450 enzymes. Synthetic chemists chase new ways to incorporate this amine motif into increasingly complex molecules, balancing scalability, yield, and regulatory requirements. Through years of optimization, R&D labs have pushed forward greener synthesis methods, such as using milder reducing agents or continuous flow reactors that limit exposure and environmental impact. These advances give regulatory bodies greater confidence in terms of worker safety and waste management.
Toxicity Research
Toxicologists have explored the risks 4-Fluorobenzylamine may pose in laboratory animals and cell lines, as fluorinated organic compounds often show distinctive metabolic pathways. Results link certain exposure levels to central nervous system effects and moderate toxicity in rodents. Enzyme inhibition has been flagged, with some reports describing transient liver changes when dosed chronically in test animals. Careful control of dosing and disposal has become part of standard laboratory practice, and ongoing studies drill deeper into long-term ecological risks from both manufacturing and end-use scenarios, aligning with increasing regulatory scrutiny.
Future Prospects
Interest in 4-Fluorobenzylamine continues to climb, not simply for its established roles but because new computational methods can now predict how tweaks to fluorinated aromatics translate to differences in biological activity or materials performance. Sectors centered on targeted cancer therapies, or novel agro-inputs for sustainable farming, see value in building more complicated fluorinated compounds from this reliable amine. Automation, digitized synthesis planning, and better environmental controls promise to further reduce risks and costs. The next wave of research probably will tap into biocatalytic methods to introduce amines and fluorines under milder, less hazardous conditions, responding to calls for greener, safer chemical processes.
A Closer Look at 4-Fluorobenzylamine
4-Fluorobenzylamine doesn’t show up in news headlines or social media debates, but its influence lingers in many lab benches and synthesis projects. The compound boasts a clear structure: a benzylamine backbone tweaked with a single fluorine atom. This seemingly small change can make all the difference in chemistry and in the wider world of products.
Shaping the World of Synthesis
Chemists reach for 4-fluorobenzylamine as a starting point in creating pharmaceuticals and specialty chemicals. The fluorine atom brings different properties from those found on plain benzylamine. These tweaks may strengthen chemical stability or change biological activity. I’ve seen colleagues spend days searching for subtle shifts in reactivity just from this kind of substitution. Drug discovery teams experiment with dozens of amine building blocks like this to unlock more effective or longer-lasting medications.
Big pharmaceutical companies invest millions hunting for molecules that can attack a disease at its core. A single fluorine switch lets chemists fine-tune how a new compound behaves in the body. Sometimes that means a medicine stays in the bloodstream longer, or it resists breaking down too quickly. Novartis and Pfizer both report success stories where selective fluorine substitution, much like what’s found in 4-fluorobenzylamine, produced drugs with better outcomes and greater safety.
Fine Chemicals Beyond the Pharmacy
Many manufacturers use 4-fluorobenzylamine to build dyes and agricultural chemicals. Specialty dyes that need sharper colors or better weather resistance often start with compounds like this. In pest management, the molecule can play a role by forming intermediates for pesticides that break down differently in soil or animals. Builders of everything from electronics to paints want cleaner, more durable results, and the underlying chemistry matters a great deal.
Risks and Responsible Handling
Strong chemicals require clear thinking and good habits. 4-Fluorobenzylamine can irritate skin, eyes, and lungs if handled carelessly. A younger version of myself once tried to skip gloves and ended up dealing with redness that didn’t fade for hours. Solvent-friendly gloves, eye protection, and fume hoods lower the chance of trouble, but some labs still cut corners to save time or cash.
On the industry side, loose safety protocols have led to reports of spills and improper disposal. That threatens water, plants, and wildlife. Regulators in the US and European Union keep watch over how companies buy, move, and use aromatic amines. Transparent reporting will force better compliance, but it takes a responsible workplace culture, not just rules, to protect workers and the environment.
Toward Safer and Smarter Chemistry
Rethinking how we build useful molecules sits at the heart of modern science. Greener alternatives—biocatalysts, milder reaction conditions, and better waste treatment—promise lower risks, cleaner air and water, and safer jobs. Industry groups and academics have begun sharing new protocols that cut down on hazards without giving up reliability or precision.
Consumers often overlook what happens on the path from raw chemical to finished product. Real progress depends on more than chemistry know-how. It takes good data, responsible leadership, and community engagement to make sure compounds like 4-fluorobenzylamine deliver benefits safely and sustainably.
Looking Closely at 4-Fluorobenzylamine
Understanding a compound like 4-Fluorobenzylamine takes more than glancing at a diagram. In the lab, I’ve worked with aromatic amines and come to appreciate how a small change can mean a new property or application. This compound carries a benzene ring, one fluorine atom, and a primary amine group. The specifics aren’t just background—these features drive its behavior, reactivity, and possible uses.
Breaking Down the Structure
4-Fluorobenzylamine’s backbone is a benzene ring, which provides stability seen in many organic molecules. Attached at the number four position is a fluorine atom, sitting opposite a “methylene amine” group. The amine itself, with its -NH2 group, connects via a single carbon (the “benzyl” portion) to the aromatic ring.
To put it in simple chemical terms, its formula reads C7H8FN. Lining up these pieces, you get a fluorine sitting in the para position, making it 4-fluoro rather than 2- or 3-. The amine group sits at the end of a short CH2 chain, not directly on the ring. This split in locations gives the molecule its own chemical flavor—electron donation from the amine can come into play, but fluorine pulls electronics the other way.
What These Details Mean in Practice
Anyone who has spent time in organic chemistry understands that one atom or a shift in group placement creates a new world of possibility. The fluorine atom, small but very electronegative, draws electrons toward itself. This shift influences how 4-Fluorobenzylamine reacts in synthesis. That’s more than academic—pharmaceutical chemistry, for example, uses these tweaks to adjust how a drug binds or behaves in the body.
The amine group plays another role. Because it’s primary and sits off the benzyl position, this makes 4-Fluorobenzylamine useful for coupling reactions and modifications, which is what attracted me the first time I worked with it. I remember mixing it in a small-scale batch, watching it serve as a stepping stone for bigger and more bioactive targets. In those moments, the theoretical world of structures becomes practical—something you can hold and measure.
Real-World Applications and Precautions
4-Fluorobenzylamine has caught the attention of many in research circles. As a building block in medicinal chemistry, it can help nudge molecules toward greater stability or selectivity by harnessing both the electron-donating and -withdrawing effects present in its structure. Adding a fluorine atom is a known strategy for tweaking solubility or slowing metabolic breakdown, which can make a difference in a drug’s real-world performance.
But these capabilities can also pose challenges. Such structures must be handled with knowledge and care; amines can cause irritation, and derivatives sometimes show up in the wrong hands. Regulations try to keep pace with misuse while not slowing down research. This isn’t just a technical hurdle—chemists like myself face choices about sourcing, documenting, and storing such compounds. Secure labs and tight records aren’t luxuries; they form the backbone of responsible research.
Building a Better Future with Careful Chemistry
Digging into the specifics of molecules like 4-Fluorobenzylamine shows chemistry’s power and complexity. Even students coming up in the lab benefit from clear models and sensible protocols. With each new variant—each swap or shift in structure—science grows. The day-to-day handling, the real chemical reactions, and safe lab practice all connect directly to the tiny differences in structure. For researchers, those details can mean the difference between success and setback.
Handling Chemicals with Care: A Matter of Safety
Most folks who work in a lab recognize the importance of having a clean and organized workspace. It isn’t just about tidiness—safely managing chemicals like 4-fluorobenzylamine makes a real difference. This colorless to pale yellow liquid has a sharp amine odor and brings with it risks if left ignored on a shelf or placed in a random container. Keeping yourself, your colleagues, and the lab itself safe starts with storing sensitive materials the right way.
Temperature: Don't Let It Get Too Warm
From personal experience, I know how easy it is to dismiss temperature control on a busy day. But 4-fluorobenzylamine loses stability if exposed to heat, and a storage slip-up in the past nearly ruined a batch we needed for an organic synthesis. Always keep it at room temperature—20 to 25°C in most labs. Some sources recommend a cooler environment, especially if you expect to store it for an extended period. It’s worth remembering that exposing this substance to high temperatures or direct sunlight can speed up decomposition and release unpleasant fumes, putting air quality at risk.
Moisture: Keep Things Dry
Water can do more damage than you might think. Moisture in a container or humid storage environment interacts with chemicals and, in certain cases, makes them behave unpredictably. For 4-fluorobenzylamine, that means using airtight bottles and desiccators, especially in humid climates or old buildings. Those of us who’ve had chemicals ruined by a leaky cap remember to double-check every time.
Compatible Containers and Labeling
Glass containers with tightly fitted caps work best. Polyethylene or polypropylene also hold up well, but glass provides a clear look at any strange sediment or color changes. Reusing containers from other chemicals could introduce traces that cause a reaction, so only use bottles cleaned for this purpose. Clear, large labels make a world of difference, especially for tired eyes during late-night experiments. Each bottle should list the concentration, date received, and hazard warnings, plus a contact in case things go wrong.
Avoid Incompatible Substances
4-Fluorobenzylamine reacts with acids, oxidizing agents, and strong bases. Storing it near bleach, nitric acid, or even some cleaning agents sets the stage for a chemical mess. Using separate shelves or clearly divided cabinets helps. After a close call with oxidizers once, I started double-checking cabinet layouts before placing anything back.
Ventilation and Emergency Readiness
Good air flow trims down the risk of vapor buildup. Labs with chemical storage should always use ventilated cabinets or rooms. In homes converted to “semi-labs,” try to avoid stashing this compound in closed closets or small fridges. If there’s any chance of a spill, absorbents and spill kits nearby save time, and knowing where to find goggles and gloves never hurt anyone.
Disposing and Keeping Tabs
Waste disposal can’t rely on wishful thinking. Used or expired 4-fluorobenzylamine gets sealed and sent to chemical waste streams—not down the drain or into the trash. Regular inventory checks help catch old stocks before they degrade and create safety hazards.
Substances like 4-fluorobenzylamine don’t forgive careless storage. A little planning and routine go a long way to protect every researcher’s health and give peace of mind to everyone in the building.
Understanding the Footprint of 4-Fluorobenzylamine
4-Fluorobenzylamine pops up in chemistry labs and some industrial setups. It serves as a building block for advanced molecules, sometimes winding its way into pharmaceuticals or research compounds. I’ve spent time around substances like this. The risks don’t just sit on paper. A little carelessness can spill over into serious consequences for both health and the wider environment.
Direct Risks to Health
Getting 4-Fluorobenzylamine on your skin or breathing in its vapors may sound harmless to someone scanning a chemical list. Look a little closer—this type of compound can irritate the skin, eyes, and even the lungs. Over time, even small exposures put you at risk for allergic reactions or worse. I’ve watched colleagues battle headaches and breathing problems after missing a glove or skipping a mask. That scene shows how fast things can go wrong.
Smart Storage and Day-to-Day Use
Proper storage makes a huge difference. Keep this chemical in tightly sealed containers, away from direct sunlight and moisture. Line those shelves with absorbent material to soak up any surprise leaks. I’ve had close calls with cracked bottles and sticky shelves. Even a single drop left behind can linger and cause issues the next day.
Personal protective gear protects you far better than good intentions. Gloves, safety glasses, and a fitted lab coat create a solid barrier. Good habits matter, too—never eat, drink, or touch your face until you’ve scrubbed up and left the area. If a spill happens, clear people out and focus on cleaning it with appropriate spill kits—never with bare hands or a t-shirt.
Ventilation and Air Quality Count
A lot of folks underestimate vapors. 4-Fluorobenzylamine puts out fumes that settle in poorly ventilated spaces. Always work with this stuff inside a chemical fume hood or near an exhaust fan that moves air the right way. I’ve felt safer even on busy days knowing that air systems pulled fumes away, not back into my lungs. Checking airflow and swapping filters keeps the setup trustworthy.
Waste Disposal with a View on Safety
Pouring leftover chemicals down the sink earned several labs I know a pile of environmental fines and lasting headaches. Set up waste containers specifically for amine-based chemicals. Label them with dates and details. Once the containers fill, call in certified chemical disposal services. Skipping this step has meant shut-downs and frightened neighbors more than once.
Training and Team Responsibility
Rules and gear only go so far if people don’t take them to heart. Regular training sessions, refreshers, and short safety meetings turn safe handling into routine rather than an afterthought. I’ve picked up best practices from mentors and peer reminders, sometimes learning more from an honest mistake than a thick manual. Encourage your team to speak up about near-misses or ideas for safer workflow.
Trustworthy Sourcing and Documentation
Buy chemicals only from suppliers who provide proper documentation, including material safety data sheets (MSDS). These sheets spell out what to expect and how to react if something goes wrong. Skipping reliable sources to save money only boosts risk on every level. I’ve watched teams face mystery spills because no paperwork came with sketchy bottles. Every shipment should match its paperwork—no excuses.
Small Steps Make a Big Difference
Building safety around 4-Fluorobenzylamine comes from habits, not just policies. Real protection grows out of choosing the right gear, staying alert, and holding each other accountable. Those steps lower risk, cut downtime, and protect everyone—lab workers, first responders, and neighbors living nearby. Nobody enjoys an accident report. Solid preparation and careful routines help keep them out of your day.
Why Purity Matters in 4-Fluorobenzylamine
Most chemists and researchers don’t waste time on materials with questionable quality. 4-Fluorobenzylamine, a staple in the toolbox for those working with pharmaceutical intermediates or organic synthesis, gets its value from purity. Only top-notch material gives reliable results, whether you’re setting up a small-scale research project or preparing a larger batch in an industrial lab.
Impurities drag down results, interfering with reactions or causing unwanted byproducts. More than once, in my career, I’ve seen new experiments go sideways because the amine source had some trace left over from a rushed synthesis or improper storage. Even a hint of a contaminant from a previous batch can spark chaos for those aiming at drug development.
Defining Purity and Chemical Grades
4-Fluorobenzylamine gets offered in a few main grades. Most suppliers provide a standard labeled “>98%” or even “99%” pure, measured by testing with gas chromatography or high-performance liquid chromatography. Each decimal point higher cuts the risk of trouble down the line. For those in pharma or biomedical research, this level often makes the difference between a mound of analytical noise and trustworthy data.
The two main chemical grades are “laboratory grade” and “analytical grade.” Laboratory grade works for school and early-stage work, but analytical or research grade suits folks reaching for publishable, reproducible results. Many European and North American suppliers highlight these distinctions because they know repeat customers demand steady, reliable quality.
Sourcing and Quality Assurance
Choosing a supplier isn’t just about seeing a high percentage on a technical sheet. Reputation helps; so does asking for a full certificate of analysis. Some labs double-check supplier claims with their own instruments, using nuclear magnetic resonance or mass spectrometry. Trust but verify—that’s kept me out of plenty of headaches over the years. If a seller shrugs off requests for analytical data or won’t answer questions about contaminants, that’s a flag worth paying attention to.
Packaging and handling matter too. 4-Fluorobenzylamine absorbs water from the air. It seems minor, until a few percent extra moisture throws off sensitive reactions. Reputable companies ship material in sealed, airtight bottles and provide storage recommendations. I’ve learned to check the bottle every time before pouring; a forgotten cap or a cracked seal signals more headaches than any paperwork.
Ethics and Traceability
The chemical supply chain often flies under the radar, but transparency keeps the process ethical and legal. Paper trails—batch numbers, lot records, and supplier certifications—provide insurance for anyone needing to prove compliance down the road. The best vendors publish this data, giving peace of mind to researchers worried about regulatory audits or product recalls.
Improving Standards
Better standards come from feedback. When labs report back on purity problems, suppliers get the pressure to upgrade processes. Government guidelines for quality control and audits from major organizations like ISO push the industry toward improved purity and documentation. Over the past decade, expectations in chemical sourcing have risen, and that overall lift benefits labs as much as the people who eventually use the technologies these chemicals help create.
Getting quality 4-Fluorobenzylamine is part science, part trust, and always informed by a personal stake in reproducibility and safety. For those dedicating time and resources to research, it pays to watch purity and grade like a hawk. In my experience, these habits save both money and credibility.