4-Ethyl-2,3-difluorobiphenyl: Tracing the Course of a Versatile Organic Compound
Historical Development
The story behind 4-Ethyl-2,3-difluorobiphenyl stretches back to the evolving toolkit of organic chemistry. Early work on biphenyl derivatives often seemed driven by practical needs in agrochemicals and pharmaceuticals. Scientists realized that swapping Hydrogen for Fluorine atoms could transform the physical properties of a molecule, leading to improved outcomes in everything from drug delivery to custom polymers. The 1980s and 1990s saw more fluorinated biphenyls being catalogued, as analytical methods matured with better chromatography and spectroscopy. The push for new liquid crystal materials put unusual biphenyls in the spotlight too, feeding a demand for synthetic routes that avoid harsh conditions or costly precursors. Today, as part of a larger toolbox, 4-Ethyl-2,3-difluorobiphenyl owes its existence to these decades of incremental improvements and a spirit of scientific persistence.
Product Overview
4-Ethyl-2,3-difluorobiphenyl is not the sort of household name you’ll find tossed around in daily conversation, but it holds steady value in research labs and specialty manufacturing. This chemical stands out once researchers or formulators look for nuanced control over solubility, reactivity, or thermal behavior. Its structural motif—a biphenyl core substituted with an ethyl group and two fluorines at key positions—distinguishes it from other aromatic compounds, giving it appeal for both experimental design and industrial experimentation. Anyone who has spent a day running NMR spectra can recognize how shifts in electron density affect downstream chemistry.
Physical & Chemical Properties
This molecule generally shows up as a pale solid at room temperature, with a melting point usually hovering around 36–41 °C. Its solubility leans toward organic solvents—think dichloromethane, ethyl acetate, or toluene—while refusing to play nicely with water, like many fluorinated aromatics. With a molecular formula of C14H12F2, it brings a molecular weight of about 218.24 g/mol. The presence of fluorine atoms doesn’t just nudge the boiling and melting points; it changes the electron landscape entirely, making nucleophilic substitution less likely, which turns out handy if a chemist wants protection from unwanted side reactions. The ethyl group at the para position? It boosts lipophilicity and can make handling a touch less frustrating compared to more volatile or reactive biphenyls.
Technical Specifications & Labeling
High-purity 4-Ethyl-2,3-difluorobiphenyl is typically supplied at 97% purity or above, with careful control of impurities that might confound analytical research or downstream processing. Reputable suppliers routinely provide an accompanying certificate of analysis, loaded with TLC purity, NMR, and mass spec results. Judging from years in the lab, a product that lands in a sealed amber vial, clearly labeled with UN numbers, batch details, and hazard warnings, signals both legal compliance and professional pride. Unlike bulk commodity chemicals, those handling specialty biphenyls cannot skip the safety data; anyone working with halogenated aromatics should always know the real story behind emergency procedures or accidental exposure.
Preparation Method
Most routes to 4-Ethyl-2,3-difluorobiphenyl still start with a cross-coupling such as Suzuki or Ullmann reactions, drawing on boronic acids and halogenated fluorinated phenyls. Careful control of temperature, solvent purity, and catalyst loading—all before lunch—often makes the difference between a high-yield product and a sticky mess on the flask walls. Some experienced chemists rely on a stepwise fluorination after assembling the biphenyl core; these days, direct fluorination using milder reagents or selectivity-enhancing ligands has become an invaluable skill. Beyond that, batch purification by silica gel chromatography and crystallization finish the process, yielding an analytical standard for both academic and industrial use.
Chemical Reactions & Modifications
This compound takes on new life in the hands of creative chemists. The biphenyl backbone offers a stage for further functionalization, often through metal-catalyzed coupling or directed lithiation. The fragrance industry and pharmaceutical developers appreciate the stability fluorine brings, which resists metabolic breakdown or photodegradation. I’ve watched colleagues try to introduce nitro or amino substituents ortho to the ethyl group, only to find the fluorines alter regioselectivity in surprising ways—proving that predicting aromatic substitutions remains more art than formula. As a precursor, 4-Ethyl-2,3-difluorobiphenyl opens doors into tailor-made ligands, custom liquid crystal materials, and designer polymers.
Synonyms & Product Names
This molecule rarely masquerades under elaborate marketing names. In catalogs, researchers see 4-Ethyl-2,3-difluorobiphenyl, often accompanied by identifying descriptors like “2,3-difluoro-4-ethylbiphenyl” or “biphenyl, 4-ethyl-2,3-difluoro-.” Some suppliers use systematized names, so veteran organic chemists pick up shorthand quickly. Keeping a chemical’s synonyms handy sidesteps confusion, especially once translated safety data or custom tariffs enter the conversation.
Safety & Operational Standards
No one in a synthetic chemistry lab shrugs off the safety profile of fluorinated biphenyls. My own training underscored how halogenated aromatics can cause skin and eye irritation or worse, depending on exposure and route of administration. Smart laboratories always stress the use of nitrile gloves, splash goggles, and refined ventilation. Handling this compound, avoid open flames and strong oxidants; even if 4-Ethyl-2,3-difluorobiphenyl does not ignite easily, respect for all chemical reagents saves lives. Disposal occurs through designated hazardous waste channels, minimizing any chance of fluorinated residues escaping into waterways. GHS labeling, supported by rigorously updated safety data sheets, provides a model all suppliers should follow—making sure everyone in the research chain feels informed and prepared.
Application Area
Chemists turn to this fluorinated biphenyl for reasons that hinge on performance in specialty synthesis, electronics, and advanced materials science. Liquid crystal formulation teams look for molecules like this when tweaking the physical response of displays and sensors. Some medicinal chemists, always looking for more metabolically stable scaffolds, have explored its core as a lead structure for new therapeutic agents. The electronics sector, hungry for molecules that resist thermal and oxidative stress, has also mapped the terrain of biphenyl derivatives. In coatings, composite plastics, and even as analytical standards, its influence pops up in patents, journal articles, and R&D projects around the globe.
Research & Development
Modern labs treat this compound as a platform, not a curiosity. Innovations in direct arylation and electrochemical fluorination feed a hunger for greener, scalable chemistry. My time spent mentoring grad students has shown that fluorinated aromatics remain a hot topic on research posters and conference talks. Teams in both academia and industry measure small tweaks in functional groups against changes in dielectric, optical, and thermal properties—a cycle that churns out better materials for screens, diagnostic tools, and chemical sensors. Journals and preprints capture these steps forward, signaling that every small win with 4-Ethyl-2,3-difluorobiphenyl unlocks potential pathways for bigger advances in design or application.
Toxicity Research
Toxicological evaluation has not kept pace with all possible use cases. Some studies on related compounds indicate moderate mammalian toxicity and persistence in the environment. Fluorinated organics, once released, resist easy breakdown; that fact alone raises red flags for environmental health. Researchers and regulators share a duty to close data gaps by funding expanded ecological and metabolic studies. My own experience in teams working with novel aromatics tells me not to take apparent stability as a sign there’s no risk. Comprehensive testing—acute exposure, chronic endpoints, biotransformation—lays the groundwork for smart use and sets boundaries to protect both people and the planet.
Future Prospects
The future for 4-Ethyl-2,3-difluorobiphenyl sits not just in new products, but in quieter revolutions in process safety, sustainability, and digital chemistry. Automated synthesis platforms and computer-guided retrosynthetic tools lower the bar to entry for young researchers building on this core structure. Companies aiming for “greener” molecules constantly trade off fluorine content with persistence in the environment, and society clearly wants materials that last without accumulating in unintended places. Stricter supply chain controls and ongoing toxicity research force the entire field to adopt higher standards, prioritizing transparency and accountability even for specialty chemicals. As regulatory landscapes shift and new uses emerge, the demands for safe handling, clear labeling, and better disposal will intensify. Every researcher or builder considering this compound in their next big project faces a challenge: use it wisely, guided by evidence and the lessons of those who came before.
Understanding the Backbone of 4-Ethyl-2,3-difluorobiphenyl
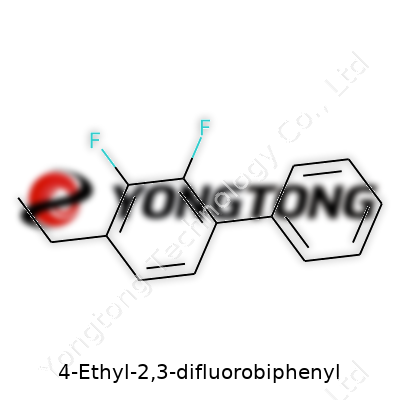
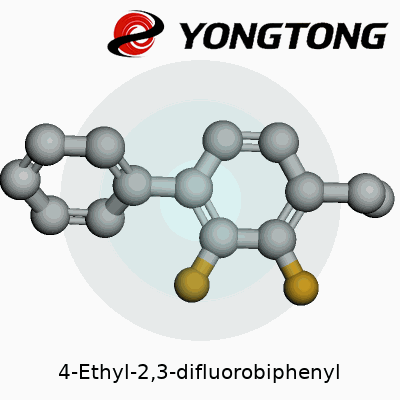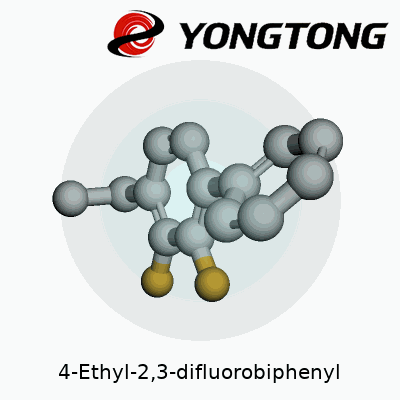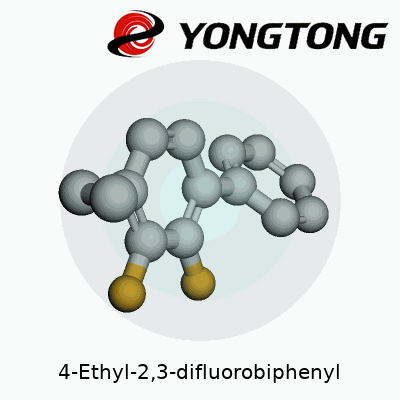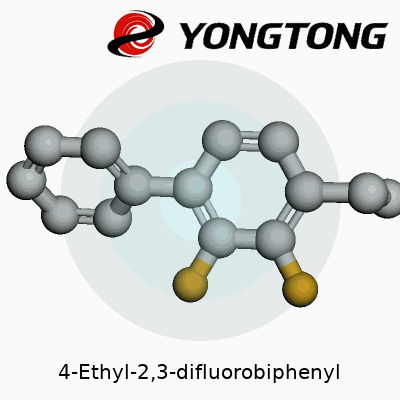
Every time I glance at a biphenyl structure, I think back to university labs with models on cluttered benches, carbons and hydrogens clicking together like grown-up LEGO. 4-Ethyl-2,3-difluorobiphenyl, to break it down, features two benzene rings. The rings join at their 1’ and 1 carbons, like two friends shaking hands. The “4-ethyl” tells us there’s an ethyl chain hanging off the fourth carbon on the first ring. The “2,3-difluoro” feature refers to a fluorine atom each attached to carbons two and three of the same first ring.
Picture the structure: benzene on the left gets that ethyl group sticking out at an angle at position 4. Two small, fiercely electronegative fluorine atoms perch like birds on positions 2 and 3, always aiming to influence the molecule’s chemistry. Across the joint, the second benzene stays plain—no added groups, simply a ring of carbons and hydrogens.
Why the Structure Draws So Much Attention
To explain the fuss, let’s talk real-world impact. Adding fluorines brings about major changes—these atoms chew up electron density, making the ring less reactive in a lot of the places folks might want to tinker with. Drop in an ethyl group, and suddenly the molecule seems bulkier, less likely to slip neatly into tight corners in a crystal lattice or enzyme pocket.
Chemists chase after this kind of modification every day. In drug research, fluorinated groups can make a molecule hang around longer in the body, or even dodge enzymes bent on chopping it up. That means better medicines—drugs that last longer, work harder, and need lower doses. Industrial chemists rub their hands together because fluorine often increases thermal stability, letting a plastic or coating stand up to abuse that would trash an unmodified cousin.
Risks, Regulation, and Solutions Worth Considering
It’s not all blue skies and breakthroughs. Plenty of well-intentioned innovations have overshot the mark. Fluorinated compounds, especially certain ones clinging to carbon skeletons, have gotten regulators’ attention in recent years. Some show up in groundwater, sticking around for decades. I recall stories from peer researchers: cleanup workers in gloves and masks, communities near legacy facilities grappling with rising levels of “forever chemicals.”
To avoid repeating history, we should talk solutions that face the future squarely. Safer laboratory practices can keep leaks and spills from starting. It’s time to invest more in green chemistry. Substituting particularly persistent chemical groups for those that can break down naturally when their industrial job’s done—this approach cuts risks before pollutants get out the door. Designing molecules with an exit plan is not just a buzzword, it reflects responsibility. The chemical structure of 4-ethyl-2,3-difluorobiphenyl can deliver rewards in research and commerce, but only if we balance those gains with open-eyed stewardship of the compounds we bring into the world.
Building a safer, smarter approach means scientists need to stay humble and keep pushing to test their innovations not just for efficacy, but for long-term impact. Sitting in a lab, poring over drawings and running chromatograms, that responsibility hangs heavy for a reason.
Breaking Down Its Place in Chemical Synthesis
Getting into any chemical compound’s list of uses might sound intimidating, but for 4-ethyl-2,3-difluorobiphenyl, its main value appears with organic synthesis. Chemists working with biphenyl cores typically chase versatility. They gravitate to compounds like this one because fluorine atoms on an aromatic ring shift reactivity and alter physical properties. Those differences can change a reaction’s outcome, which matters most while developing new substances for medicines, materials, or agricultural purposes.
A solid example: chemists in pharmaceutical labs don’t just swap out hydrogens for fluorines on a whim. Fluorines dial up metabolic stability, tune electronic effects, and, sometimes, sharpen selectivity against disease targets. This habit has led to more than 20% of commercial pharmaceuticals now containing at least one fluorinated group. 4-ethyl-2,3-difluorobiphenyl lands in the toolkit of those developing intermediates for more advanced compounds. Some of its derivatives can show up in active pharmaceutical ingredient pipelines, although regulations demand rigorous testing.
Materials Science: Adjusting Performance with Subtle Tweaks
4-ethyl-2,3-difluorobiphenyl also gets attention from material scientists searching for improved polymers, OLEDs, and specialty coatings. Here, the position of ethyl and fluorine groups can influence torsional angles between the rings, shifting melting points and thermal stability. I’ve watched lab teams run battery after battery of thin-film tests because a few atoms’ change can mean brighter displays, longer life, or more efficient solar cells.
Fluorine’s presence reduces surface energy, which tends to boost resistance to stains or the environment. For anyone who’s ruined a favorite shirt with a stray drop of coffee, these small benefits hit home. Those who design next-gen screens or textiles look for ways to embed such chemical features from the molecular level up.
Building Blocks for Agrochemical Research
Chemistry doesn’t stay in the lab. Agrochemical developers reach for biphenyl compounds like 4-ethyl-2,3-difluorobiphenyl for leads in new pesticides and fungicides. Small shifts in chemical structure can translate to much higher activity or greater environmental persistence. While some critics worry about lasting residues, proper stewardship and rigorous field studies aim to keep any new agent safe for both farmer and ecosystem.
Fluorinated biphenyls sometimes help improve selectivity, hitting weeds or pests harder without damaging crops. A decade ago, I joined a project mapping soil runoff after using a fluorinated herbicide; the optimized structure mattered. Without that detailed design work in the lab, field results can quickly send developers back to square one.
Weighing the Challenges
Tougher regulations on fluorinated chemicals keep research teams on their toes. Disposal of byproducts and safe handling both shape how quickly new methods move forward. My own experience tells me that transparency helps most—sharing full life-cycle data with regulators and open publication of safety outcomes can build trust.
Many of today’s bright ideas trace back to modest biphenyl compounds. The way professionals pick apart and rebuild these molecules explains why some solutions break through in medicine, tech, or farming. For 4-ethyl-2,3-difluorobiphenyl, just a few tweaks at the drawing board can ripple far down the line.
Why Purity Grades Matter in Real-World Applications
Anybody who works around specialty chemicals knows that purity is much more than a number on a certificate. With 4-Ethyl-2,3-difluorobiphenyl, the advertised grade typically reaches 98% or higher. That sounds impressive, and for most industrial and research projects, it fits the bill. But beneath that high percentage lies a reality—impurities always hitch a ride, even if you can’t see them.
Researchers in pharma, electronics, or advanced materials notice abrupt headaches when purity slides below what sensitive projects demand. Side reactions run wild, yields drop, and time goes straight down the drain. As someone involved in setting up custom syntheses, I’ve learned that skipping on early purity checks nearly guarantees expensive repeat work and head-scratching troubleshooting. A figure like “98% GC” used to sound airtight, but I’ve learned to ask, “Where’s the other 2%?” That sliver can sometimes include leftover catalysts, solvents, or by-products that don’t play nice with tight-lipped chemistry.
How Purity Impacts Safety, Scale, and Ethics
Lab staff running larger quantities recognize contamination early, sometimes by smell, sometimes by the unexpected fizz of a reaction. Even trace residues can blunt the effectiveness of a product, or worse, set off unpredictable hazards. Purity isn’t snobbery—it’s about keeping people safe and projects moving. Refineries and manufacturers feel this on their bottom line. If 4-Ethyl-2,3-difluorobiphenyl enters a pilot plant below promise, it risks off-batch production costs, equipment cleaning, or even forced shutdowns for quality control violations. Major recalls have their roots in ignored decimal points.
There’s also a growing ethical side. Product recalls mostly end up in the news, but internally, failed experiments or rejected lots mean wasted resources—solvents, energy, labor. Responsible chemical suppliers publish detailed Certificates of Analysis, often using high-end techniques like GC/MS or NMR, not just high-performance liquid chromatography, to assure buyers of the absence of hard-to-detect contaminants. This isn’t just regulatory ticking; it’s about real accountability.
Solutions and Best Approaches for Buyers and Suppliers
The conversation doesn’t end at 98%. Buyers who value their downstream work need to push for batch-level data—no skipping the paperwork. Trace impurity profiles give life to those extra decimal points. In my experience, direct communication works. Ask the supplier for solid confirmation: How was purity measured? Is the grade “as supplied” or “after drying”? How recent is the batch analysis? A reputable seller won’t hesitate to clarify.
If the stakes run high, request a small sample before placing a bigger order. Run your own analytics or partner with a local lab. This isn’t about mistrusting the supplier but double-checking that the material suits your specific reaction or formulation. Sometimes, labs need something higher—say, 99+%—for specialized synthesis, where even the tiniest residues can break a pathway. Custom purification can be arranged if you ask, and, yes, it often adds to the cost, but it may save plenty of grief later.
Across industries, a solid supplier relationship makes honest discussions around purity routine. It’s not just about chasing the highest figure on a datasheet. It’s about having the facts, making decisions with clear eyes, and knowing that even 2% can change the story in the lab, on the production floor, and in the final product.
Storing Chemicals Is No Time for Shortcuts
In any lab, a new chemical bottle lands on the shelf and folks ask, “Where should this go?” It might seem simple, but there’s a lot at stake. 4-Ethyl-2,3-difluorobiphenyl doesn’t look scary—clear crystalline, no wild fumes, not an acid that burns through gloves in seconds. Yet, smart storage keeps everyone safe and avoids expensive mistakes.
What Makes 4-Ethyl-2,3-difluorobiphenyl Special?
This biphenyl derivative pops up in organic synthesis and research. The fluorine atoms can make it stable, but stability on paper isn’t always what you see in real life. Many biphenyls stick around for ages but can turn dangerous if ignored, neglected, or accidently mixed with things they never ought to meet.
Storing for Lab Safety—and Science Integrity
Many researchers fall back on the old rule—store cool, dry, and dark. That advice works for a lot of compounds, but not every detail gets captured with catchall phrases. Based on experience in academic and industry labs, the biggest risks come from temperature swings, light exposure, and loose handling.
Heat speeds up reactions most of us don’t want. Sunbeam through a window, radiator in winter, forgotten heat lamp—these can push small chemicals like 4-Ethyl-2,3-difluorobiphenyl to break down or react. I watched colleagues learn this the hard way: a batch went bad over the summer before anyone noticed.
So, shelf space away from heat, not near HVAC vents or windows, keeps things from drifting into the danger zone. A temperature log nearby helps, especially for rare or pricey samples. Most reference guides put the upper limit safely below 30°C, so ordinary room conditions usually fit the bill.
Moisture: The Silent Trouble Maker
Humidity trips up more storage plans than most realize. Even chemicals that look solid and harmless can pull in water, changing how they react or how pure they stay. Silica gel packs or a well-sealed desiccator cut down this risk. Every time someone fumbles with the cap or lid and leaves it half-closed, the odds of spoilage go up. Tip: Teach labmates the habit of closing containers right away, before any distractions set in.
Keeping Chemicals Where They Belong
Mixing incompatible chemicals starts out as an oversight but ends with ruined materials or worse, an emergency. 4-Ethyl-2,3-difluorobiphenyl sits best with other organics, not oxidizers, strong acids, or bases. Add a clear label on the storage bottle—every user, no exception.
Checking for Change—Keep an Eye Out
I check the bottle every month or so, looking for clumps, yellowing, or leaking. Anything new means it’s time to check the literature or safety data sheet, sometimes even send a small sample for analysis. Ignoring these signs gets expensive fast: lost experiments, lab shutdown, maybe even a fire risk.
Leading with care—good labeling, checking temperature, staying dry—keeps this compound ready for research, not for a spill report. Take these steps, and both the lab and the science keep moving forward.
Finding a safety data sheet for something like 4-Ethyl-2,3-difluorobiphenyl shouldn’t feel like chasing a phantom. That’s a mouthful of a name, but it matters more than it sounds for people who actually use or work around it. Tossing chemicals around in labs or factories isn’t possible without solid info on how to handle them, what spills can do, or how it might mess with your body. As someone who’s handled his share of unfamiliar compounds, the search for safety data isn’t just academic—it can keep you out of the ER or worse.
The Hunt for Reliable Safety Data
Go online, search databases like Sigma-Aldrich, PubChem, or ChemSpider, and you might come up blank. Lots of small-batch or new custom chemicals get made quicker than regulatory info follows. This one doesn’t pop up with a clear SDS from any major supplier as of early 2024. That makes life hard for anyone responsible for lab safety or regulatory compliance. Most universities, and plenty of manufacturing outfits, just won’t let a chemical on-site without its SDS. OSHA rules don’t fudge much here either. Without that sheet, stuff like safe storage temperature, required PPE, and fire fighting measures become a guessing game. Not a good spot to be in.
Why This Hits Beyond the Lab
Small molecules like biphenyl derivatives matter in everything from pharma projects to materials research. That means grad students, bench chemists, and even janitorial crew can get exposed. Years ago, I walked past a spill cleanup where the crew only had advice scribbled on a sticky note because the actual SDS hadn’t arrived. That sort of scenario makes the risks real. Unknowns around toxic fumes, waste disposal, or reactivity push people into risky choices. Mistakes happen when key data is missing. Unfortunately, that can add up to medical leave, lab downtime, and permanent health impacts.
Ideas That Put Safety First
Lack of an SDS for a new or obscure molecule like this signals a wider problem: chemical supply chains still have some shadowy corners. Some contract labs do a basic hazard screening, but that doesn’t give anyone enough to write a complete SDS. Labs working with unknowns end up patching together info from analog molecules, but that gets sketchy fast. It’s not much help if your compound catches fire while its closest relative just smolders.
Research groups, and small-scale chemical suppliers, could work together to share hazard data in open-access databases. Even a shared, crowdsourced summary beats total silence. Digital platforms like PubChem and ChemRXiv already let people post spectra or synthesis routes—adding real-world safety observations would help fill gaps before manufacturers catch up. Suppliers selling new or uncommon chemicals might build in an SDS-writing service for every product ordered, even if it ups the final price. That levels the playing field for small labs and big companies alike.
Final Thoughts on Filling the Data Gap
Anyone tasked with chemical safety can feel frustrated by regulatory gaps, but waiting for slow-moving agencies or paperwork to catch up isn’t safe. Push toward more open sharing of chemical hazards—between universities, suppliers, and end-users—cuts down risk. Clear, honest reporting as soon as a new chemical hits the workbench helps out the next person in line. Nobody wants to play roulette with something that even Google can’t describe.