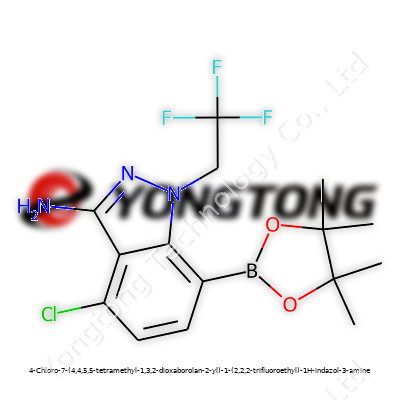
4-Chloro-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-indazol-3-amine: Deep Dive into a Modern Chemical Cornerstone
Historical Development
Chemists have grappled with the challenge of functionalizing indazole for decades. The evolution of cross-coupling technology in the late twentieth century, followed by a boom in boronic ester chemistry, opened fresh ground for complex substitutions on heterocycles. This compound came into the picture as researchers pushed to harness the combined signatures of fluorination and boronic acid derivatives. By the time industry leaders transferred reaction protocols from lab notebooks to scaled production, the focus on chemoselectivity grew, pushing synthetic chemists to develop milder catalysts, better ligands, and more robust purification techniques. It’s hard to overstate its impact, since it drew on years of collective know-how in halogenation, selective borylation, and fluorine chemistry rooted in pharmaceutical, agrochemical, and materials fields.
Product Overview
4-Chloro-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-indazol-3-amine offers a striking example of how careful design can shepherd a molecule into multiple markets. Its scaffold packs a substantial punch: a chloro substituent for selective reactivity, a boronic ester arm fit for Suzuki-Miyaura couplings, an indazole ring system prized for its biochemical potential, and a trifluoroethyl handle that changes metabolic stability and lipophilicity. Labs chasing new kinase inhibitors or probing unknown binding pockets rely on this blend of properties to explore chemical space.
Physical & Chemical Properties
With a white to off-white solid appearance, this compound clocks in at a molecular weight of about 398 g/mol. The combination of boronate and chloro groups influences solubility—expect it to dissolve in common organic solvents like DMSO and DMF, less so in water. The presence of the trifluoroethyl group bumps up hydrophobic character, altering chromatographic behavior and partitioning, which matters when laying out purification protocols or bioavailability studies. In NMR spectra, the molecule tells its own story: boron shifts, sharp fluorine peaks, clean aromatic signals, plus amine functionality ripe for derivatization.
Technical Specifications & Labeling
Suppliers will typically ship this product with a purity exceeding 97%, often verified by HPLC, and moisture content below 0.5%. On the vial, you’ll spot hazards tied to skin or respiratory exposure, with batch number, synthesis route identification, and storage conditions—usually tightly sealed, cool, and dry. Advanced stocks include QR code tracking for regulatory compliance, which comes in handy for audits or custom synthesis triggers. Labeling strictness matters, especially as regulatory regimes on boron compounds have tightened globally. In the lab, a quick scan of the label reveals solvent compatibility, shelf-life, and supplier contact details, making inventory management easier and reducing error.
Preparation Method
Getting to this molecule often starts with a chlorinated indazole backbone. Using palladium-catalyzed borylation, chemists attach the pinacol boronic ester under inert conditions—argon or nitrogen lines, checked for leaks at every step. The trifluoroethyl group enters through nucleophilic substitution, carefully timed to sidestep overreaction or unwanted side products. Amination at position-3 calls for protected intermediates, followed by selective deprotection using acid or base, depending on the functional group landscape. Scale-up runs benefit from flow chemistry setups, monitoring temperature jumps to avoid runaway reactions, especially under exothermic borylation. Each phase involves TLC and LC-MS crosstalk to verify purity, catching lags before the final workup.
Chemical Reactions & Modifications
Synthetic chemists value this compound as a crossroads for elaboration. The boronic ester reacts smoothly in Suzuki couplings, letting researchers bolt on aryls, heterocycles, or vinyl groups from their modular libraries. The chloro group supports selective substitution by nucleophiles—sulfur, oxygen, or nitrogen-centered—without scrambling the rest of the molecule. The amine end welcomes acylation, sulfonylation, or even diazotization, laying groundwork for photochemical or bioconjugation experiments. The stability of the trifluoroethyl substituent keeps side reactions in check during most transformations, which cuts manual labor at the bench and curbs costs for scale-up.
Synonyms & Product Names
Trade catalogues and journals sometimes swap in shorthand or systematic labels: the compound appears as 4-Chloro-7-(pinacolboryl)-1-(2,2,2-trifluoroethyl)-indazol-3-amine, PINACOL boronate indazole, or by research code designations in medicinal chemistry pipelines. Chemical Abstracts indexes it under its full IUPAC descriptor, while suppliers create quirky catalogue codes—CBTI-6110 or TFE-Indazole-BPin, depending on the house style. Navigating this tangle means checking registry numbers and supplier metadata before placing an order, since synonyms can mask subtle differences in purification or salt form.
Safety & Operational Standards
Handling this compound means running through a familiar checklist: nitrile gloves, goggles, and lab coats serve as non-negotiables, while working inside a ventilated fume hood guards against dust inhalation or offgassing. Containment protocols rise in priority as the trifluoroethyl group could break down under heat, releasing small amounts of toxic vapor. Emergency data sheets warn against open flames, state guidelines for neutralizing small spills with inert absorbers, and stipulate waste disposal routes to satisfy both local and international guidelines on boron and halogenated byproducts. Regular training on chemical hygiene, chemical spill response, and up-to-date inventory logs reduces mishaps and keeps personnel prepared, as required by regulatory agencies.
Application Area
Major drug discovery hubs deploy this compound as a building block in synthesizing kinase inhibitors, cytokine modulators, and signal transduction agents. The boronic ester functionality turns it into a lynchpin for fragment-based drug design, showing up in early-stage SAR campaigns and hit-to-lead projects. Agrochemical teams test these frameworks for crop protectants, hoping to tailor membrane permeability with the trifluoroethyl tag, while polymer chemists see potential in speciality coatings. Academic groups extract value from this molecule as a springboard for novel heterocycle research, patterning away from the standard indazole lineage to chase uncharted receptor targets or artificial enzyme platforms.
Research & Development
Collaborative research drives continual redesign of synthetic steps, often seeking greener catalysis or sharper selectivity. Cyclization tricks, directed ortho-metalation, and novel ligand classes cut down on waste and lower barriers to custom analog generation. Analytical teams keep refining their toolkit with high-field NMR, advanced mass spectrometry, and single-crystal X-ray diffraction, pegging every detail of structure and reactivity the moment a new batch gets prepared. Intellectual property filings cite new application claims—not just as synthetic intermediates, but as active moieties in their own right.
Toxicity Research
Toxicologists turn their attention to both acute and chronic effects, mapping distribution and metabolic fate in model organisms. Preliminary testing on cell lines shows a low baseline for cytotoxicity, but the combination of halogen and trifluoromethyl groups prompts extra scrutiny for persistence in the environment. Animal model work tracks oral and dermal LD50 values, benchmarks organ distribution, and flags delayed excretion. Environmental safety regulators watch for boronic acid runoff from production sites, checking wastewater for residuals before sign-off on manufacturing permits. Lessons from this class of molecules inform better solvent selection and encourage closed-loop manufacturing.
Future Prospects
Looking to the future, more industries plan to pick apart both the core indazole and its functionalized arms. Machine learning tools already scan chemical spaces for derivatives optimized for binding affinity and ADMET properties, linking new motifs to the parent molecule. Process chemists look at digital reaction monitoring and AI-powered prediction of side products, aiming to reach kilogram or tonne scales with fewer reworks. As demand for fluorinated, boron-containing scaffolds rises, especially for targeted therapeutics and advanced sensors, supply chain partners and regulatory agencies debate the safest and most sustainable pathways from kilo labs to pilot plants. The molecule continues to spur creative collaborations between chemists, biologists, and engineers who seek not just new drugs, but smarter ways to make them.
Why Purity Matters in the Lab and Industry
A lot of chemists find themselves focused on structure and function, but purity checks shape the very outcomes of their work. In academic labs, people often look for upwards of 97% purity before pushing compounds into further studies. If contaminants slip in, even at the last decimal point, unpredictable results aren’t far behind. Synthetic organic chemistry walks a line where traces of starting material, solvents, or side products feel like loose wires in an electrical system. Problems aren’t just theoretical. I’ve had reactions stall and chromatograms look like city skylines, and tracing the culprit usually lands on a purity slip.
Industrial chemists put just as much attention on these details, because real-world uses demand tight tolerances. Legal registration for new pharmaceuticals, agricultural chemicals, or materials insists on certificates of analysis. These reports nail down exact amounts of the target compound—say, 98.4%—along with named impurities identified through HPLC or LC-MS. Batch-to-batch consistency turns theoretical interest into real safety and performance. If a pilot scale batch comes in with only 88% purity, reformulation or purification eats into time, cost, and patent deadlines.
What Analytical Data Reveals
Looking at 4-Chloro-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-indazol-3-amine, the complexity signals a need for careful checking. Boronic esters often run into hydrolysis, so even a speck of water during isolation can split the dioxaborolane ring. Fluorinated fragments bring their own stubborn impurities, as incomplete fluorination leaves similar-mass byproducts behind. Depending on the synthetic route, leftover copper, palladium, or even small fragments from silica gel might sneak into the final product. I’ve faced these in more than one project—every small mistake stands out under the wrong column or mass scan.
Sourcing from major chemical suppliers, you can expect high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) results with each vial. These tell the story in peaks, integrals, and chemical shifts. Genuine analytical data offers transparency; 99.1% reported purity, for instance, means less doubt. If supplier data glosses over these details, alarm bells go off for both the eager undergraduate and the seasoned process chemist.
Pushing Toward Solutions
To safeguard experimental outcomes, in-house testing before use remains a standard move. Quick checks—TLC, NMR, melting point—sometimes reveal what a glossy datasheet hides. On big projects, outsourcing to a trusted analytical lab, or collaborating on method development, stops problems before they scale up. Researchers push for suppliers that share full supporting spectra and impurity profiles, instead of just numbers. Open access to data lets everyone verify claims, swap best practices, and learn from failures.
Environmental, safety, and global compliance shape every conversation about purity. Nobody wants banned solvents, ghost peaks, or toxic metal traces showing up late in the development process. Regulatory bodies ask tough questions about each contaminant, and labs that keep detailed purity records have proof ready when the time comes. Quality control runs deep—every small bottle represents work, money, and trust.
Why Storage Matters More Than Most Think
Anyone who’s worked in a lab or stocked a pharmacy shelf realizes chemicals have a mind of their own. One week, a compound looks fine—just a plain old powder in a sealed bottle. Next thing you know, humidity creeps in, labels fade, caps stick, and the whole batch gets tossed. That’s about more than inconvenience. Once, I stored an oxidizer near an open window, thinking the breeze would keep things cool. The substance clumped and caked within days. The cost? Lost time, wasted money, and a stern reminder from a safety officer. Whether in research, healthcare, or industry, reliable storage keeps everyone safer and saves budgets from headaches.
The Enemy: Heat, Light, and Moisture
Three villains cause trouble: heat, light, and moisture. Storing most chemicals works best at standard room temperature, roughly between 20°C and 25°C. Anything hotter can speed up decomposition, invite chemical changes, or set off reactions. Basements get humid; think twice before using them for sensitive materials. Direct sunlight? Worst spot for most compounds, as UV rays drive breakdowns and discoloration.
I once ignored a label and left a vial of a sensitive dye on a sunny shelf. By day three, what was bright blue turned pale green—useless for experiments. Manufacturers warn about these things, and for good reason. So, keep chemical bottles away from sunny windows and radiators. A sturdy cabinet or even a plain wooden box in a shaded corner will do far more good than high-tech setups, unless the substance demands chilling or freezing.
Humidity: The Silent Saboteur
Water vapor in the air sneaks into every crack and makes a mess of powders and hygroscopic materials. Store those in tightly-sealed bottles, preferably glass, with proper gasket lids. Toss in a silica gel pack if you’ve got one. Years ago, after losing expensive reagents to clumping, I started double-bagging bottles and keeping a cheap humidity logger nearby. Tracking moisture levels isn’t overkill; it only takes one ruined shipment to prove the point.
Avoiding Cross-Contamination
Mixing incompatible chemicals in close quarters creates risks most folks overlook. Most acids dislike bases, and oxidizers hate being stored with organics. It pays to divide shelves by classes, if space allows. In training, I learned from seeing alkaline cleaners stored right beside bleach—a mix-up that sent two workers home early. The lesson stuck. Pay attention to chemical compatibility charts.
Labeling and Routine Checks
Proper labeling beats even the sharpest memory. I use waterproof markers and date every container the day it opens. Routine checks on expiry dates and seals take minutes. I’ve caught degraded compounds just by noticing color shifts or odd odors; these little inspections matter for quality and safety.
Digital Reminders and Practical Solutions
Many now use digital inventory systems. They flag reordering points and keep logs of storage conditions. This isn’t about high-end technology for its own sake; even basic spreadsheets or notebook logs boost accountability. Investing in basic temperature and humidity monitors pays off in fewer spoiled batches.
Training: The Best Safeguard
Staff education stands as the key defense. Even the toughest storage guidelines fail if no one understands why they exist. Bringing new team members up to speed, sharing stories of near-misses, and reviewing protocols go beyond rule-following—they help foster a culture where good storage habits become second nature. Years of experience taught me that a little vigilance up front spares a lot of grief down the line.
Why a CoA Matters in Today’s World
A Certificate of Analysis, or CoA, represents more than just a checklist item. In the industries I’ve worked with—food, supplements, chemicals—nobody likes surprises. You want to know what’s going into your product or what’s landing on your table. A CoA works like a detailed scorecard for every batch that leaves a manufacturer’s doors. It shows data on purity, potency, and the absence of contaminants. For folks with allergies or specific dietary needs, that means peace of mind. For companies, it makes recalls less likely, lawsuits avoidable, and reputations less shaky.
One time, a local supplement brand in my city found itself scrambling: the pills were contaminated with traces of a heavy metal. The root cause—sloppy supplier verification and missing CoAs. In another example, a juice importer I knew switched suppliers to save a few bucks, only to receive products with pesticide residues well above the legal limit. No CoA, and within weeks, their shelf space vanished in every major grocery store around.
What a Good CoA Tells You
A real CoA does not read like mystery instructions. It lists actual measurable details: what the product is, the batch or lot number, and all the test results. You find clear statements like “contains less than 0.3% lead” or “passed microbial testing.” Dates matter. So does the name of the lab and the signature of a qualified analyst. If any of this is missing, it’s a red flag.
Trustworthy suppliers don’t dodge questions about CoAs. They understand this document’s power. In growing regulations for supply chains and consumer goods, smart businesses ask their suppliers for current CoAs as a standard practice. If a supplier resists, buyers either walk or risk joining the headlines for all the wrong reasons.
Demand Driving Higher Quality
One trend stands out: consumers are far more informed than ever. They read labels. They Google product origins. Many ask for a Copy of the Certificate of Analysis before they buy. I’ve seen this play out at farmers markets, online wellness groups, and even in family grocery chats. The growing call for proof is forcing brands—big and small—to step up.
Reputable producers now post CoAs online, especially in the hemp and CBD space. Some software companies have cropped up to help smaller growers share real-time batch data with their buyers. Grocery stores ask for them, too, especially if they want to stay out of regulatory messes. The more often folks insist on seeing a CoA, the less chance contaminated or mislabeled products creep onto shelves.
Real Solutions for Stronger Trust
If retailers and manufacturers carry products with their name attached, they can require the latest batch-level CoAs with each shipment—not just the first order. Labs should be accredited, and CoAs should show the actual lab’s address and credentials. Tech can automate much of the tracking. Digital QR codes on packaging that link straight to a CoA have become more common, and they make checking ingredient claims quick and transparent.
These changes build trust—one batch of protein powder, wine, or baby food at a time. The fewer secrets in the supply chain, the better off we all are.
What Science Looks Like in the Lab
Navigating chemistry in the real world starts with the basics—knowing the molecular formula and molecular weight. These two bits of information tell you everything about a compound's raw makeup and the mass you’ll see on a balance. To a researcher trying to replicate results or a pharmacist calculating dosages, precision here is non-negotiable. My own early days as an undergrad, mixing reagents in a cramped teaching lab, taught me that simple mistakes in weight or formula could turn a scheduled experiment into a late-night troubleshooting session.
How Chemists Find the Molecular Formula
The journey to pin down a molecular formula always runs through analysis. Most folks in a lab reach for analytic tools—mass spectrometers, nuclear magnetic resonance, X-ray crystallography. These tools help figure out the pure numbers of atoms and their arrangement. For everyday tasks, you break a compound down into its elemental parts—carbon, hydrogen, oxygen, nitrogen, and so on. Tallying these up gets you the molecular formula. That short string of letters and numbers (such as C6H12O6 for glucose) gives a snapshot of what’s there. It translates into something any chemist in the world recognizes.
Molecular Weight Matters Beyond the Textbook
Molecular weight tells you how heavy a single molecule weighs in atomic mass units (amu). Add up the atomic weights of each atom in the formula and you have your answer. That value winds up stuck on every bottle in a lab fridge and every sheet of safety data. Forget accuracy here, and your work sinks. Whether scaling up a synthesis for industry or crafting personalized medicine in a hospital’s cleanroom, the outcomes rest squarely on getting this figure right.
Real-World Impact and Avoiding Pitfalls
I’ve seen surprising mistakes come just from sloppy math or outdated reference books. At one point, a colleague lost a valuable batch of material by using an old formula scribbled on a bench top. These moments drive home the point—accurate, updated data builds trust within teams and across industries. Scientists catch errors by double-checking with up-to-date sources like PubChem or the Merck Index, both industry standards for chemical information.
Solutions and Steps Forward
Keeping labs and factories safe comes down to more than memory or trusting scribbled notes. Many companies set up strict protocols where two people cross-check any calculation, whether for a small analytical test or a large-scale batch. Sometimes, it means building digital tools into daily workflow—a molecular calculator, access to digital databases, or automated barcode systems for tracking compounds and weights. Smart software and collaborative checking catch errors before they escape the lab.
In my own experience, laying out straightforward documentation and making it clear for everyone—new hires, senior chemists, visiting scholars—removes confusion. It helps avoid the small missteps that ripple into major setbacks, protecting research and, in the bigger picture, patient safety or clean processes in manufacturing.
Why These Details Matter
Get the formula or weight wrong, and nobody trusts the result, whether you’re working in basic research or producing a lifesaving drug. Attention here reflects deeper respect for science, teamwork, and the people counting on those calculations being right. The world outside the lab sees only the outcomes—but for every experiment or drug that works, getting that earliest calculation correct is what makes the entire process real, safe, and repeatable.
Cutting Through the Noise in Research and Everyday Labs
Every lab worker knows the search for a new product kicks off a set of questions. What can this really do? Does it solve a real problem or just sit on the shelf? For researchers, these answers either push a project forward or waste precious hours. Over the years, having worked across both academic and industry labs, I’ve seen how the right reagent or device can shave months off a timeline, help publish stronger data, and even keep a team together when frustration and deadlines creep in.
Sharpening Results in Applied Science
Chemistry, biology, and materials science keep evolving. Teams run experiments on tighter budgets, and pressure to innovate never lets up. Products with true value often pack a punch by saving steps or removing frequent sources of error. For instance, advanced reagents that offer consistent particle sizes, high purity, or stable compounds simplify tasks from synthesizing nanoparticles to screening drug candidates. In cell biology, having reagents that guarantee low endotoxin levels cuts down on spurious results, so work moves without repeat experiments. My past projects in protein purification underlined the cost of questionable batch quality. Reliable supplies trimmed downtime, letting us focus on breakthrough work, not troubleshooting.
Helping Environmental and Food Safety Labs
Testing keeps our water, soil, and food safe. In environmental labs, I’ve used products that enhance sensitivity in detecting trace metals or organic toxins. Volatile chemicals, harsh extraction steps, and low detection limits demand solid performance from every tool. Having consumables that hold up under these stressful setups means less worry about contamination or false readings. In a food safety role, consistent assay reagents ensured public health inspectors trusted the results we sent out. Anything that reduced human error or offered clear color change for fast interpretation now gets my immediate attention.
Bringing Value in Medical Diagnostics
Medical researchers, both in academia and biotech startups, lean hard on reliable standards and calibrators. During my stint supporting diagnostics, having quality controls with traceability, certified by outside agencies, took much of the stress out of assay development. Products making it easier to spot disease markers, with clear and consistent reactivity under varied conditions, cut down repeat runs and made approval processes smoother. Since laboratory errors cost both time and money—sometimes with patient lives in the balance—every upgrade matters. Good controls, easy-to-handle packaging, and clear labeling all pay dividends in busy clinical labs.
Paving the Way for Sustainable Research
Sustainability isn’t just a buzzword. Green chemistry and safer lab protocols drive product adoption now more than ever. Supplies with lower environmental impact—either requiring less hazardous waste disposal or coming in recyclable materials—have become a priority in my purchasing decisions. Across several departments, switching to these alternatives came with fewer regrets about the bigger picture, and suppliers who prove their sustainability claims with clear documentation always won us over.
Meeting the Needs of Rapid Prototyping and Education
In teaching, ease of use stands out. Educators need experiments that work out of the box and fail gracefully if things go wrong. Anything packaged with clear instructions, resistant to common handling mistakes, and adaptable across skill levels earns repeat use. Undergraduates learn best when chemistry and biology succeed on the first try, not when frustration kills curiosity.
Driving Innovation, Not Just Another Sale
Companies justified their price only when products led to cleaner, faster, or more reliable results. A product that slashes troubleshooting builds real trust. Whether doing advanced spectroscopy, food screening, or teaching high school science, I’ve always come back to suppliers that offer practical, proven performance.