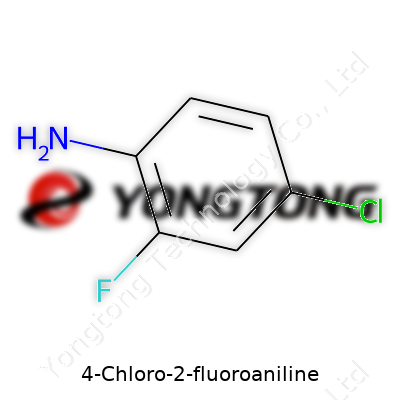
4-Chloro-2-fluoroaniline: Its Path Through Science and Industry
Historical Development
Back in the early push for new synthetic intermediates, the chemical world caught onto the usefulness of halogenated anilines. Among them, 4-chloro-2-fluoroaniline emerged as a standout, first cropping up in patent records tied to dye production and expanded stepwise into pharmaceuticals and agrochemicals. The drive to replace more hazardous or inefficient aromatic amines in the 1970s and 1980s put this compound on the radar for both big labs and scrappy startups. Researchers appreciated how a single ring could open so many doors, tweaking properties one halogen atom at a time. The chemistry community recognized its potential quickly, both in Europe and Asia, after early suppliers scaled up production. This practical value ensured its spot as a backbone intermediate across several innovative processes.
Product Overview
4-Chloro-2-fluoroaniline draws interest as a raw material and an intermediate, not just a stockroom staple. It offers a unique set of reactivity patterns because of its two halogen substitutions on the aniline ring. This specificity grants manufacturers flexibility and precise control—when crafting new pesticides, pharmaceuticals, or specialty dyes, handling a compound like this lowers the risk of unintended byproducts. Often found as a solid powder or a crystalline material, it ships worldwide, with industrial partners in fine chemicals trading on reliability and purity, not just price per kilogram. For companies building multi-step synthesis pathways, this compound sits at key junctions where selectivity and stability make or break efficiency.
Physical & Chemical Properties
The molecular formula, C6H5ClFN, makes clear that 4-chloro-2-fluoroaniline balances simplicity with diverse reactivity. Boiling just around 210°C and melting near 28-30°C, it avoids complications with storage or shipment—no need for low-temperature logistics. It forms off-white to pale yellow crystals under normal conditions. Water solubility remains low, underscoring its need for careful handling in waste treatment streams, but it dissolves readily in organic solvents such as chloroform, ether, and alcohol. That helps chemists weave it easily into more complex transformations. Its electron-withdrawing substituents lower reactivity at the ortho and para positions, tuning its behavior during coupling reactions and allowing scientists to target specific molecular modifications.
Technical Specifications & Labeling
Industry and regulatory bodies set specifications for 4-chloro-2-fluoroaniline purity, calling for an assay (by GC) usually higher than 98%. Common labels feature manufacturer lot numbers, purity percentages, and standard hazard warnings. Safety data sheets underline health risks and storage guidelines, and proper labeling requires seeing hazard symbols for skin and eye irritation and environmental impact. Drum and container documentation supports shipment traceability, all the way from large-scale industry to small research labs. Purchasing teams look for certification to standards like ISO 9001 or GMP compliance, both for supply chain integrity and to streamline audits or regulatory file buildup.
Preparation Method
Manufacturers rely on halogen exchange and selective substitution methods, taking advantage of established aromatic substitution reactions. Typical procedures start from 4-chloro-2-nitroaniline or similar derivatives, using fluorinating agents such as potassium fluoride and a catalyzed system—copper(I) salts often get the nod. After fluorination, reduction of the corresponding nitro compound through catalytic hydrogenation leads to the target amine, which is purified by recrystallization or vacuum distillation. Each step gets tuned for scale, with some plants focusing on continuous flow reactors to dampen batch-to-batch variability and minimize waste. Local environmental rules inform choices over solvents and waste treatment, pushing some producers to greener protocols in recent years.
Chemical Reactions & Modifications
4-Chloro-2-fluoroaniline frequently serves as a precursor for further aromatic substitution, nucleophilic aromatic substitution, and coupling reactions. The amine function opens the door for acylation, sulfonation, and diazotization. In pharmaceutical and agrochemical synthesis, it provides a base motif for more complex substituent patterns, giving researchers an efficient starting point for structure-activity relationship (SAR) studies. Chemists can introduce alkyl, aryl, or heterocyclic groups through Buchwald-Hartwig or Suzuki-type cross-coupling, the halogen atoms serving both as activation sites and functional handles. This compound’s stability under a range of reaction conditions gives process developers room to experiment, reduce process steps, and improve atom economy.
Synonyms & Product Names
Across catalogs and research papers, 4-chloro-2-fluoroaniline shows up under a handful of aliases. Chemists use systematic names like 2-fluoro-4-chloroaniline, as well as shorter trade names or internal codes in supplier listings. The variety complicates procurement, especially across language or regulatory regions—consistency in those records prevents costly mix-ups. Checking chemical structure diagrams or using CAS numbers (like 364-26-5) remains the gold standard, particularly for multinational projects or supply chain verification. A single base molecule, many names, all pointing to the same industrial workhorse.
Safety & Operational Standards
No one cuts corners with aromatic amines—4-chloro-2-fluoroaniline demands strict respect in the lab. Safety teams reinforce PPE requirements: gloves, goggles, and effective ventilation all come standard. This compound can irritate skin, eyes, and breathing passages, with chronic exposure risks if used carelessly. Many manufacturers operate under REACH or TSCA rules, keeping detailed exposure and hazard logs. Fire risk remains low under normal storage, but reaction mishaps during large-scale synthesis—think runaway exotherms—push labs to build robust containment and response systems. Spill protocols and secondary containment aren’t just paperwork; they keep workers and downstream communities safe. In practice, storage in cool, dry spaces with clear demarcation for incompatible materials, such as strong acids or oxidizers, underpins safe chemical management.
Application Area
Industries choose 4-chloro-2-fluoroaniline where selectivity and product purity can drive profits and innovation. In the crop protection field, it acts as a stepping stone to insecticides, herbicides, or fungicides that battle resistance and raise yields. Pharmaceutical formulators favor it in parts of synthetic drug design, particularly where fluoro- and chloro-groups tune metabolic stability or modulate biological activity profiles. Dye and pigment makers use the compound as a building block for high-performance colorants, installing robust chemical motifs into complex molecules that must last in sunlight or harsh processing environments. Nearby, advanced material science teams in electronics, polymers, and specialty adhesives keep finding new corners of the marketplace for such halogenated amines.
Research & Development
R&D teams look past the basic utility of 4-chloro-2-fluoroaniline, tapping into its versatility as a fine chemical tool. Ongoing work focuses on greener, less wasteful synthesis strategies, including transition metal-catalyzed routes and solvent-free conditions. Researchers at universities and specialty chemical makers track modifications in reaction efficiency, atom economy, and carbon footprint, hoping to meet both rising regulatory demands and client sustainability goals. In medicinal chemistry, teams push structure-activity loops, seeing incremental tweaks in the starting scaffold translate into dramatic shifts in pharmacology. This compound anchors combinatorial chemistry libraries, generating data for next-generation computational models that predict what pharmaceutical candidates will succeed in the clinic or what agrochemical innovations can overcome new pest challenges.
Toxicity Research
Toxicologists treat halogenated aromatic amines with heightened skepticism, running cell line, animal model, and aquatic toxicity tests to stay ahead of regulatory shifts. 4-chloro-2-fluoroaniline can cause acute irritation and sensitization, with some chronic exposure studies indicating a need for occupational monitoring. Data suggest pathways for bioaccumulation and potential breakdown into more reactive intermediates, especially under high-temperature or oxidative industrial conditions. Eco-toxicology screens report low to moderate persistence, prodding producers to shore up end-of-life waste and emissions controls. Vetted, peer-reviewed studies underpin workplace exposure limits set by multiple national authorities. Clear communication of risks, updated with emerging literature, makes sure that labs and processors stay prepared for tighter environmental and worker safety standards down the line.
Future Prospects
Looking out over the next decade, demand for specialty building blocks like 4-chloro-2-fluoroaniline will likely keep rising, pressed by evolving pharmaceutical targets and changes in global agriculture policy. Digital chemistry and AI-driven molecular design lift the importance of well-characterized, modular intermediates, exactly what this molecule offers. Sustainability initiatives put pressure on manufacturers to revisit classic synthetic routes, axing hazardous reagents and reducing process waste. Cleaner fluorination methods and more selective catalytic transformations promise not just environmental gains, but also better cost control and consistency. As regulations tighten and market needs shift, success will depend on balancing technical performance with transparent safety documentation and robust supply chain records. Researchers and industry pros will keep finding new molecular spaces to explore—always looking for compounds like this one that offer reliability, tunability, and a proven safety track record.
Roots in Agrochemicals
Farmers know that pests and weeds wipe out crop yield. Chemists spend countless hours turning molecules into solutions for this problem. 4-Chloro-2-fluoroaniline, with its halogenated aniline structure, often shows up at the start of that journey. It stands out because it can form strong bonds with other chemicals, helping scientists build new compounds tailored for specific pest-fighting jobs. Plenty of herbicides and insecticides on the market trace their origins to molecules like this one. The chemical backbone it offers gives serious flexibility in tweaking other parts of the molecule, so chemists get a shot at better targeting weeds and bugs that threaten food supply.
Dyes and Pigments Manufacturing
Vivid, lasting colors show up in plastics, textiles, and printing ink. At the core of many dye molecules, you’ll find compounds derived from aniline or its relatives. Brands demand colorfastness—they want shades that stand up to sunlight and regular washing. 4-Chloro-2-fluoroaniline offers chemical stability that anchors dye molecules, making it possible for manufacturers to deliver the dependable colors customers expect. That tough structure comes from its halogen atoms. These atoms help the dye resist breaking down over time, so it sticks around on fabric or plastic much longer than older dyes did.
Pharmaceutical Ingredient
Many modern medicines start with small, versatile organic molecules. Drug makers often grab 4-Chloro-2-fluoroaniline early in the synthesis of certain antihistamines, anti-inflammatories, and even cancer therapy candidates. It lets researchers add new groups to the molecule, which tweak how the eventual drug works in the body. Its specific blend of chlorine and fluorine gives medicinal chemists a way to adjust everything from drug metabolism to how well a compound binds its intended target. The pharmaceutical industry chases efficiency, and reliable building blocks like this one mean shorter development timelines and lower costs, without cutting corners on safety.
Building Block for Advanced Materials
The world keeps asking for materials that can survive harsh treatment—whether it’s in an aerospace part, medical device, or electronics. Chemical engineers reach for 4-Chloro-2-fluoroaniline when designing specialty polymers and resins. Adding it into a polymer chain can help tune properties like resistance to heat and corrosion. Factories crafting coatings or adhesives for demanding environments use derivatives of this molecule to keep surfaces protected and functioning for longer.
Potential Solutions and Responsible Handling
The demand for faster, safer, and more sustainable solutions continues to grow. Careful design in synthesis and downstream use matters a lot here. Cutting-edge research is giving chemists new routes to the same end molecules, but with less toxic waste and lower emissions. These greener manufacturing approaches do more than check a regulatory box—they protect workers and communities, and reduce cleanup costs down the road.
Strict regulations surround anilines because of health and environmental risks. Personal experience working in chemical labs taught me that good planning and solid process controls aren’t just recommended—they’re vital. Using closed systems, proper training, and continuous monitoring keeps people safe and prevents accidental releases to the environment. Transparent documentation and accountability help everyone—managers, workers, and neighbors—sleep better at night.
Looking Forward
4-Chloro-2-fluoroaniline will keep its place in chemical research and production because its unique structure opens doors. As regulations tighten, companies must invest in cleaner processes and safer handling. The next advances will likely come from the intersection of chemistry, engineering, and environmental science—making sure progress in one field doesn’t set us back in another.
Understanding 4-Chloro-2-fluoroaniline
4-Chloro-2-fluoroaniline often finds its way into research labs and specialty chemical catalogs. Its chemical formula, C6H5ClFN, reveals a structure typical of aromatic amines, with subtle tweaks that make it worth a second look. By adding a chlorine atom at the fourth position and a fluorine at the second, chemists tailor its properties for specific uses. It’s not just another aniline derivative you stumble across in textbooks—for synthetic chemists and analysts, every slight modification matters.
Why Do Formula and Molecular Weight Matter?
You might think the chemical formula is just a set of letters and numbers, but it drives so much of what we do in the lab. Take C6H5ClFN—one chlorine, one fluorine, a nitrogen, and that classic six-carbon backbone of benzene. This lineup lets you predict behavior in reactions, compatibility with reagents, and even how it needs to be stored. Molecular weight, another basic but crucial detail, lets you calculate how much of a substance you need for a given reaction. For 4-Chloro-2-fluoroaniline, the molecular weight lands at 145.56 grams per mole.
Early in my days working with chemical syntheses, I learned the hard way that skipping the calculation led to big problems. We once wasted a whole afternoon because nobody bothered double-checking the molecular weight in a sequence. Scaling a reaction from milligrams to grams demands more than a casual guess. You can’t just eyeball it and hope for the best.
Role in Modern Chemistry
This compound doesn’t just serve as a textbook example. Use of halogenated anilines like 4-Chloro-2-fluoroaniline continues to grow in pharmaceutical research. Chemists value these slight tweaks on well-known structures since they often lead to new biological activity—a fresh lead for drug discovery or an intermediate for agrochemicals.
Safety matters, too. With halogens in the mix, properties shift: new hazards might pop up, so those working with this molecule need to consult up-to-date safety data every time. Chlorine and fluorine aren’t friendly elements to play with. I remember reviews that highlighted cases where poor ventilation made a routine synthesis much riskier. Reliable protocols save more than just the product—they can save your skin.
Solutions and Best Practices for Working With 4-Chloro-2-fluoroaniline
Chemical suppliers and academic labs need to make the information around formula and weight easy to access. This isn’t just about ticking a regulatory box—it’s about trust and reproducibility. Labs should make sure up-to-date datasheets are on hand and that researchers know how to verify the identity and purity of their chemicals before use. It pays to set up training so new researchers can double-check formulas and weights before every experiment.
When you know exactly what you’re working with, from the molecular formula down to the origin of your chemicals, you avoid bad surprises. Mistakes usually don’t come from complicated parts of the job—they sneak in from things that seem too basic to check. It’s not about just following rules. It’s about building habits that keep work on track and results reliable.
Hazards Give Clear Instructions, Not Options
Every lab tech remembers the sting of learning about chemical hazards the hard way. 4-Chloro-2-fluoroaniline looks like just another white powder on the shelf, but trust me, it demands respect. The compound carries toxic and possibly mutagenic risks, so direct skin contact or breathing in its dust is a gamble nobody should take. Read through any safety data sheet and you’ll catch the same message—this compound can irritate skin, damage organs, and pollute the environment. Making excuses for shortcuts ends up painful or expensive.
Lock It Away—Don’t Settle for “General Storage”
Shelf space isn’t just about convenience with chemicals like this. 4-Chloro-2-fluoroaniline needs a completely dry, tightly sealed container, out of direct sunlight. Temperature shifts break stability and create more risk—especially if humidity drifts up. I’ve seen bulk supplies go bad from nothing more than a cracked lid or careless shelving near a heat vent. Use high-density polyethylene or amber glass containers. Clear labeling matters, too. A faded sticker leads to someone grabbing the wrong jar, or worse, skipping personal protection.
Respect PPE—Leave No Exception
Chemical-resistant gloves and eye protection come first. No one wants to learn the hard way how nasty a splash to the face or a surprise rash can get. Laboratory coats with long sleeves and closed footwear should always be used. Respiratory protection steps in during weighing or mixing, as airborne dust sneaks up on the distracted. I’ve watched even careful chemists skip a step under pressure and regret it—tightly following personal protection protocol always pays off.
Decent Ventilation Makes All the Difference
Few people realize how quickly an enclosed space can fill with noxious chemical vapor or dust. Small labs sometimes try to save on fume hood costs, but any work involving 4-Chloro-2-fluoroaniline belongs inside well-functioning local exhaust ventilation. Fume hoods trap dust and vapors, making accidental inhalation far less likely. Keeping the airflow strong and regular equipment checks prevent unexpected buildup. Trying to weigh or transfer chemicals outside a controlled area raises injury risk. One sting in the back of your throat makes ventilation’s value obvious.
Failsafe Disposal Avoids Regret Later
Down-the-drain disposal belongs in the past. Contaminated glassware and unused solids need collection as hazardous waste. Local environmental authorities usually outline rules for toxic aromatic amines like this one. My advice: use sealed, shatter-proof containers for disposal, and keep everything labeled by date and contents. Document all disposal steps in a simple logbook—even small spills deserve logging. Teams that communicate about disposal mistakes suffer fewer disasters.
Training and Teamwork Keep People Safe
Fresh hires or graduate students won’t absorb safety habits just by watching others. Routine, specific training—alongside practical drills—teaches skills that posters and written rules do not. I’ve seen labs where simple habits, like changing gloves between steps or double-checking seals, made all the difference after a spill. Build a culture of accountability and nobody hesitates to speak up or fix a missed step.
Building a Safer Routine Starts with One Compound
Managing chemicals like 4-Chloro-2-fluoroaniline well sets a tone for every other substance in the storeroom. Strong habits around labeling, PPE, and ventilation scale easily, protecting people, equipment, and records. Everyone wants to work in a place where safety is visible in every decision, not just a line in the company manual.
What Makes This Chemical Stand Out
Few people spend their day thinking about industrial chemicals like 4-Chloro-2-fluoroaniline. Yet anyone working near dyes, agrochemicals, or pharmaceuticals faces a higher risk of exposure. Companies use this compound as a building block, so it finds its way into production lines, shipping containers, and factory waste.
Why Health Professionals Are Concerned
Anilines cause a stir among toxicologists for good reason. The chemical structure lets them disrupt the body at a cellular level. Breathing in dust or vapors, getting the stuff on your skin, or even swallowing tiny particles all carry big risks. Data from peer-reviewed studies point to several ways exposure can go wrong.
Red blood cells face the first hit. 4-Chloro-2-fluoroaniline, like other aniline derivatives, encourages the build-up of methemoglobin. This mutated form of hemoglobin can’t transport oxygen as it should. Symptoms often follow rapidly—think headache, shortness of breath, dizziness, even a blue tint to lips and fingers. In high doses, it can spiral into convulsions or organ damage. For someone with an underlying health problem or reduced lung function, even small exposures strain the system.
Impact Goes Beyond Short-Term Symptoms
Years ago, I watched coworkers handle chemicals with little more than rubber gloves. Some believed their quick exposure did no harm. New research paints a different picture. Longer-term skin contact can mean persistent rashes or allergic reactions. Some research even links prolonged exposure to possible cancer risks, although these findings remain unsettled in the wider literature.
Personal experience in busy lab settings tells me most workers can’t always avoid contact. Accidents happen. Spills soak into fabric and shoes. Without prompt washing, chemical burns develop or the toxin slips through the skin, entering the bloodstream. Data from poison control centers highlight how unpredictable the aftermath can be—some walk away with irritation, others need emergency care for serious poisoning.
Environmental and Occupational Hazards Stack Up
The risk doesn’t stop at human health. Industrial leaks or poor storage often mean chemical runoff. Once this compound slips into wastewater, it presents a threat to wildlife. Fish and amphibians show altered growth and behavior, based on field studies from areas with repeated releases. Local residents drink water drawn from the same sources, so exposure sometimes extends to entire communities.
In my days consulting for manufacturing plants, poor ventilation kept hitting the top of my worry list. Open drums, poorly labeled containers, or basic dust masks do little to filter out airborne toxins. Factory floors with high turnover rarely get thorough training on chemical hazards. That’s one reason why stricter regulations matter.
How People and Workplaces Can Respond
Anyone working near 4-Chloro-2-fluoroaniline needs quality protective equipment and a system for rapid spill response. Upgraded ventilation keeps air cleaner. Regular blood testing helps catch rising methemoglobin before it gets dangerous. Engineering controls, such as closed systems, cut down on leaks and vaporization.
If your workplace stores or ships this compound, up-to-date hazard communication systems make a world of difference. I’ve seen big improvements after companies swapped outdated binders for digital alerts and routine briefings. Investing in staff training leads not only to fewer accidents, but fosters a culture where people watch out for each other. My own experience says that feeling empowered and informed saves lives as much as any piece of safety gear.
Why Purity Grades Matter
Walking through a chemistry lab, one lesson always sticks: the real work begins before anything hits the beaker. The purity of chemicals like 4-Chloro-2-fluoroaniline draws a bold line between successful results and wasted time. A lower-purity batch throws off reactions, introduces new contaminants, or leaves you scratching your head at mystery peaks on an HPLC trace.
Lab suppliers don’t ship everything in one standard grade. Step into a catalog, and the choice of purities hits you right away, each with its own sticker price and set of expectations. Some batches reach up toward pharmaceutical or analytical standards—99% or higher—while others land closer to technical grade, a little less expensive, often used in bulk manufacturing or tests where fine margins don’t matter.
How Purity Origins Impact Real Results
Chemists seeking reproducibility trust higher-purity reagents. This comes from experience, not just convenience. A single impurity at 1% can become a headache down the line, sometimes blocking regulatory approval or skewing toxicology work. That’s a hard lesson for anyone who’s seen months of work unravel over what started as a “good enough” batch.
A team working on pharmaceuticals will demand documentation with every order, sometimes even a certificate listing detected trace impurities, water content, or residual solvents. For 4-Chloro-2-fluoroaniline, this difference in grade isn’t theoretical—it’s tied directly to patient safety, environmental risks, and lab budgets. Commodities shipped to a dye plant, on the other hand, rarely need that level of scrutiny.
Supply Chains and Purity Choices
Behind these differences sits a complex supply chain. Producers synthesize 4-Chloro-2-fluoroaniline to laboratory, industrial, or pharmaceutical grades using various purification steps; fractional distillation, crystallization, chromatography. Each step carves out impurities and increases price. The technical grade likely comes with fewer cleanup steps, but for researchers developing a drug or evaluating precise analytical methods, cutting corners brings more risk than reward.
Suppliers carry different grades to meet needs across industries. Companies with strong reputations always publish purity certificates, sometimes called Certificates of Analysis (COA), making verification easier. These documents—once just page filler—are now considered a minimum requirement in regulated industries.
Real-World Decisions and Practical Solutions
Cherry-picking the right grade has always been a balancing act between cost and end use. A chemical engineer scaling up a process might run initial experiments with a lower-purity option. Once results start looking promising, the switch to purer material avoids repeating the costly mistake of letting impurities kill a project later on. Over the years, this practice saves both money and headaches, especially if the ultimate goal involves clinical trials or regulatory approval.
In research, students or startups sometimes try to cut costs by buying technical grade material, thinking the risk is low. Anyone who has tried this route knows it often backfires—troubleshooting odd reactions burns more hours than the price difference.
Labs need easy access to supporting documentation and clear labelling. Transparent catalogs—those that honestly list impurity profiles—make smart buying easier. Without that, researchers gamble with both time and safety. Teams in regulated fields find peace of mind in trusted vendors who back up claims with actual test data.
Building Blocks and Future Focus
With sustainability and green chemistry gaining ground, more buyers want sustainable manufacturing practices and information on waste produced during purification. Audit trails that document purity from source to shipment help customers verify not just chemical quality, but also ethical sourcing and environmental safeguards.
So, the next time someone scans options for 4-Chloro-2-fluoroaniline, the range of purity grades available does more than offer variety. It serves labs demanding precision, organizations watching budgets, and researchers who rely on robust and safe science to solve pressing problems.