4-Butyl-3-Fluorobiphenyl: A Commentary
Historical Development
Chemists have followed the trail of biphenyl derivatives for more than a century, primarily through their impact on medicinal and materials science. Interest in fluorinated biphenyls picked up in the late twentieth century, and 4-butyl-3-fluorobiphenyl entered the scene as researchers realized the importance of tweaking the biphenyl backbone with both alkyl and fluorine groups. This work followed a broader movement in organic chemistry, where tweaking molecules led to new drugs and smarter materials. I remember reading an early synthesis paper from the 1990s that framed substituents on biphenyl rings not as decorations, but as ways to shift electronic distribution, change metabolic outcomes, and nudge properties like solubility and thermal stability. Today, 4-butyl-3-fluorobiphenyl marks an intersection of careful organic synthesis and the growing toolbox for pharmaceutical discovery.
Product Overview
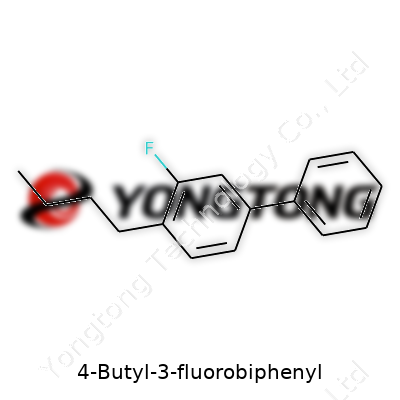
4-Butyl-3-fluorobiphenyl looks like a modest aromatic hydrocarbon at first glance. There’s the signature biphenyl—two benzene rings connected—but the substitution stands out: a butyl chain sitting at the 4-position, altering the compound’s hydrophobic profile, and a fluorine atom hanging at the 3-position, known to make molecules more resistant to metabolic breakdown. Every time I’ve handled such molecules, I notice the extra weight fluorine brings, often a sign of someone thinking about drug-likeness. Even outside the drug world, modifications like this turn up in liquid crystal formulations, specialty monomers, and target compounds for advanced electronics use.
Physical & Chemical Properties
You get a crystalline solid under ambient conditions; this type of biphenyl derivative doesn’t fuss much about humidity, holding its shape and chemical integrity on the bench. The butyl group helps the compound dissolve in nonpolar organic solvents—ether, toluene, and cyclohexane all work—yet it won’t budge in water. Its melting point typically runs higher than simple biphenyl, owing to both increased mass and fluorine’s impact on intermolecular forces. Chemists appreciate its chemical stability around air and mild bases. The molecule resists acid-driven hydrolysis, and the aromatic core provides a buffer against most oxidizing agents. You also find that the fluorine atom alters reactivity patterns at the adjacent positions, often blocking further electrophilic substitution near its spot on the ring.
Technical Specifications & Labeling
Producers who sell 4-butyl-3-fluorobiphenyl pay close attention to purity, often guaranteeing 98% or higher. Clear labeling calls out its molecular formula (C16H17F), exact mass (about 228.31 g/mol), and warranted absence of halogenated byproducts or heavy metal contamination. I have yet to see regulatory agencies in North America or Europe flag this compound for special handling, but producers typically print hazard phrases pointing to mild skin or eye irritation and strongly recommend use of gloves and goggles. Shipping labels point out the organic, non-water reactive character. Most vendors offer documentation such as COA (certificate of analysis) or SDS (safety data sheet) alongside the product.
Preparation Method
Synthesis of 4-butyl-3-fluorobiphenyl draws on the well-choreographed Suzuki–Miyaura coupling, a staple in modern labs. In practice, I recall charging a round-bottom flask with a halogenated biphenyl (often 3-fluorobromobenzene) and a boronic acid (such as 4-butylphenylboronic acid), adding a palladium catalyst, and running the reaction in toluene or DMF at 70–100 degrees Celsius. You get clean cross-coupling after a few hours, separate by column chromatography, and sometimes finish with a distillation or recrystallization. The mechanism—palladium oxidative addition, transmetalation, and reductive elimination—shows up across countless textbooks. What matters is careful handling of the air- and moisture-sensitive catalysts, and patience at the purification step, as similar biphenyls often show up as close-eluting side products.
Chemical Reactions & Modifications
Once synthesized, 4-butyl-3-fluorobiphenyl stands up to further chemical games. The butyl side chain can be oxidized—slowly—using mild oxidants, or trimmed back via radical bromination followed by elimination. The biphenyl core can take part in directed ortho-metalation, where chemists add groups next to the fluorine. That’s been handy in the lab for building more complicated molecules, with the fluorine acting almost like a signpost for where to react next. Halogen–metal exchange opens doors for functionalizing both rings, and the entire molecule sits as a versatile intermediate for other biphenyl-based fine chemicals and pharmacophores.
Synonyms & Product Names
Anyone shopping for this compound may come across a few alternate handles. Common synonyms include 3-Fluoro-4-butylbiphenyl, 4-Butyl-3-fluoro-1,1'-biphenyl, and BP4-3F among others. Databases and vendors sometimes flip the positions, but checking the systematic name keeps confusion at bay. Chemical supply houses register the product under its CAS number, and some list it in catalogs aimed at pharmaceutical R&D or specialty chemical intermediates.
Safety & Operational Standards
Handling 4-butyl-3-fluorobiphenyl in the lab comes with standard organic safety: work in a fume hood, keep food and drinks away, wear chemical splash goggles, nitrile gloves, and a lab coat. Accidental exposure generally causes skin or eye irritation rather than systemic harm, but no one wants to take the risk lightly. Most users store the compound tightly capped, away from oxidizers and ignition sources. The low volatility minimizes the risk of inhalation, but chronic studies on operator exposure are lacking—so most labs err on the side of caution. Chemical waste goes into halogenated organic bins, never down the drain. In larger-scale facilities, spill kits and chemical-vapor scrubbers are part of the norm.
Application Area
Labs put 4-butyl-3-fluorobiphenyl to use in multiple ways. In drug discovery, small modifications like butylation and fluorination can flip a molecule’s biological behavior. I’ve watched teams explore analogues as leads for anti-inflammatory or antineoplastic agents, tweaking substituents to push for increased potency or metabolic stability. The compound’s rigid planar structure makes it a candidate for advanced materials—liquid crystal displays, in particular, draw on biphenyl derivatives to fine-tune optical switching. Polymers using functionalized biphenyls as blocks achieve higher glass transition temperatures and mechanical toughness, which matters in cutting-edge electronics. The same backbone shows up in dye chemistry, UV stabilizers, and even rare fragrance bases. Application keeps expanding as more research unlocks structure–activity relationships.
Research & Development
Research around 4-butyl-3-fluorobiphenyl keeps finding new territory. Medicinal chemists talk about the power of single-atom swaps—a fluorine for a hydrogen—turning weak binders into real candidates, all while sidestepping enzyme degradation issues common to simpler aromatics. Studies build SAR (structure–activity relationship) matrices, shifting the butyl group or moving fluorine around the ring. Physical chemists model liquid phase behavior, especially where fluorinated biphenyls change display properties or push the boundaries of organic electronics. In my own work, we’ve mapped NMR and mass spec fingerprints to track tiny modifications, critical during quality control of scale-up batches.
Toxicity Research
Publicly available data on outright toxicity shows moderate concern. Like related biphenyls, acute toxicity runs low, with oral LD50 in rodents usually above 1000 mg/kg. No major carcinogenic or mutagenic effects pop up in standard panels, but studies remain limited due to its relatively niche status. The main concern circles around long-term bioaccumulation and breakdown products. Some fluorinated aromatics resist environmental degradation—a red flag for persistent organic pollutants. Most precautions target skin contact and inhalation, as chronic exposure lacks comprehensive study. Institutions urge responsible disposal to limit accidental release, and chemists pay attention to the potential for persistent halogenated byproducts both during manufacture and degradation.
Future Prospects
4-Butyl-3-fluorobiphenyl sits right in the overlap between synthetic chemistry and tomorrow’s materials. Its structure enables new analogues for therapies that tackle drug resistance, all by shifting one side chain. Electronics research points to further expansion in displays and nanomaterials, especially as greener synthesis reduces environmental concerns. I’d like to see open sharing of toxicological data and best-practice protocols to keep emerging halogenated aromatics safe throughout their life cycle. Collaboration between medicinal, environmental, and industrial chemists will drive smarter applications—balancing function and safety long after researchers move on to newer biphenyl derivatives.
Chemistry Behind the Scenes
Walk into any lab focused on creating new medicines or advanced plastics and you’ll see shelves lined with all sorts of small aromatic compounds. 4-Butyl-3-fluorobiphenyl catches the eye among these chemicals because of its structure. Chemists use the biphenyl backbone as a reliable “building block” in both research and production. Adding groups like butyl and fluorine tweaks how the molecule behaves, letting researchers guide it toward certain uses.
Pharmaceutical Roots and Research
Talk about biphenyls in drug discovery, and you’ll find plenty of enthusiasm. These compounds pop up often as scientists build potential medicines. The butyl and fluorine in 4-Butyl-3-fluorobiphenyl matter for more than just their long names. The butyl group changes how the molecule interacts with fats and water, while fluorine adds strength and can block enzymes from chewing up the compound too quickly. This combination gives researchers a better shot at creating drugs that stick around in the body and target tricky enzymes.
I’ve seen teams use similar molecules to design everything from painkillers to treatments that go after stubborn cancers. While 4-Butyl-3-fluorobiphenyl itself may not land on a pharmacy shelf any time soon, its role as a starting point or intermediate can’t be underestimated. Changing one group or linking it with another fragment opens the door to a whole new class of molecules.
Materials Science and Electronics
The electronics industry leans on biphenyl compounds for more than just small molecules in test tubes. With precise adjustments, they become building blocks for liquid crystal displays or organic light-emitting diodes (OLEDs). That fluorine atom, even in small concentrations, influences how well the material withstands heat and handles electrical currents. Butyl’s presence makes the structure more flexible or changes how these compounds organize themselves in thin films.
Scientists chasing new display or sensor technology leverage these detailed changes. By tweaking the composition, they reach for sharper images, lower power use, or longer-lasting screens. You’ll rarely see 4-Butyl-3-fluorobiphenyl by name in consumer brochures, but it can help create layers that do the heavy lifting inside the devices we use every day.
Environmental Safety and Handling
Labs keep a close eye on safety when working with biphenyl compounds. Some of these chemicals linger in the environment or show toxicity with careless use. Proper ventilation, gloves, and careful waste handling matter as much as the research itself. Regulatory agencies stress the need for disposal protocols that protect both people and ecosystems.
Manufacturers and academic researchers have stepped up to develop greener synthesis routes and look for alternatives with lower impacts. Even small tweaks in production can cut waste or make clean-up easier. Sharing these improvements through workshops or scholarly journals spreads good practice across the field.
Developing Chemical Know-How
The road from a simple aromatic compound in a catalog to the heart of a new drug or device stretches on for years. Every step counts: from choosing a compound for study, to scaling up, to meeting safety standards. 4-Butyl-3-fluorobiphenyl reminds us that science relies on lots of trial, error, and collaboration. Progress comes from building on small discoveries—layer by layer, molecule by molecule.
Understanding the Chemical Layout
4-Butyl-3-fluorobiphenyl isn’t a household name. Even in scientific circles, talking through its composition helps folks see the big picture. This compound belongs to the biphenyl family—two benzene rings locked together, sharing a single bond. Now picture a butyl group (a straight chain of four carbon atoms) tagging on at the number 4 position of one ring, and a fluorine atom hitching to the number 3 spot on the other. Chemists describe it with the formula C16H17F, where the two bulky rings make room for both extras: the butyl chain juts out, while fluorine’s halogen strength brings an edge.
What Makes its Shape Significant
In my days working alongside researchers in organic chemistry, the way we see a molecule influences how we handle it. A biphenyl backbone doesn’t just offer structure—it shapes properties, like melting point and how the molecule interacts with light or dissolves in solvents. Tacking a butyl group onto the fourth carbon puffs up the molecule, changing how it fits or stacks with others. Add that fluorine at the third position, and you deepen resistance to breakdown. Fluorine makes the structure more stable, sticks stronger to carbon, and boosts heat resistance and lipophilicity. In lab tests, these tweaks often affect how the compound moves through the environment or the human body.
Where Real-World Uses Come In
Research on biphenyls stretches far. People in the chemical industry often look at biphenyl-based structures when searching for new liquid crystalline materials, which power the screens on phones and TVs. That butyl chain—a nonpolar tail—helps the compound dissolve in oils and boosts flexibility in how molecules align in a thin layer. Meanwhile, fluorine imparts chemical stability that helps these materials last longer under heat or UV.
In manufacturing, the specific arrangement in 4-butyl-3-fluorobiphenyl opens up custom tweaks for specialty coatings and advanced composites. The compound’s structure fits into the world of materials with high mechanical strength and thermal reliability. Still, every tweak brings new questions—toxicity, persistence in water and soil, and the possibility of bioaccumulation.
Balancing Progress with Responsibility
My experience with chemical safety taught me that new compounds, even those with proven benefits, bring responsibilities. Fluorinated compounds draw attention for their environmental impact. Perfluorinated chemicals, distant cousins to this molecule, stick around in groundwater and have prompted tough action from regulators worldwide. Each new fluorine-bearing molecule deserves scrutiny.
Researchers and companies who want to use or create molecules like 4-butyl-3-fluorobiphenyl should set up long-term toxicity and biodegradation studies. It’s also worth involving local communities early when manufacturers bring in new compounds. Strong monitoring keeps the environment and public health in view. In my work, open data and sharing results with peer reviewers and community leaders foster trust and let innovation continue at a steady clip. Chemistry shapes the world, but people decide what’s worth making.
Understanding the Hazards
Most people outside a lab never hear about 4-Butyl-3-fluorobiphenyl. Chemists and process engineers may cross paths with it on certain projects, but its hazards look no different from dozens of similar substituted aromatics. This is not table salt. If you’ve smelled or accidentally touched a halogenated organic, you know these aren’t benign materials. Exposure may irritate eyes, lungs, or skin. Nobody wants to deal with those burning, raw sensations during a shift. In some cases, the risks run deeper—chronic exposure to biphenyl derivatives can pose longer-term health threats, including organ toxicity.
Personal Protective Equipment Actually Matters
Not long ago, I watched a new lab tech dismiss the need for proper gloves while measuring this compound. In minutes, she regretted it; her skin showed red splotches, and she felt dizzy. No shortcut saves you from basic precautions. Good gloves—nitrile or butyl—keep these chemicals off your skin. Splash goggles help guard your eyes. A lab coat, preferably with sleeves that fit tight at the wrist, creates a barrier for arms and torso. If you know solvents or powders are in play, then a full-face shield and chemical apron take things up a notch. Even if gloves feel cumbersome, nobody has ever said, “I wish I hadn’t protected myself.”
Control Those Fumes
Many folks underestimate the way volatile compounds like 4-Butyl-3-fluorobiphenyl can hang in the air. It does not take much for inhalation exposure to become a problem, especially if you’re weighing or transferring it. An effective fume hood isn’t a suggestion here—it’s the foundation. Airflow protects not just your lungs, but the entire workspace. Years ago, I saw a minor spill outside of a hood lead to a persistent cough across the lab. Ventilation and fume containment are your first defense before even reaching for a respirator. If a spill happens beyond the hood’s barrier, evacuate and ventilate. Don’t hope for the best—air out and stay out until it’s cleared up thoroughly.
The Art of Safe Handling
Spill management isn’t a theoretical skill—it’s a habit. Before handling 4-Butyl-3-fluorobiphenyl, lay out spill kits with absorbent material, gloves, and waste bags. Use dedicated tools for weighing and transferring. Clean up every grain and rinse worktops with proper solvents. Label every bottle clearly, not just with the compound name but also hazard warnings; confusion never helps anyone. Even a professional can mistake one clear solution for another on a busy day. Keeping a regular habit of double-checking containers and logging chemistry runs pays off. That means less cleanup, fewer exposures, and less time wasted hunting down what happened.
Keeping Training Fresh
Decades of experience in labs show the value of routine safety refreshers. One overlooked step, and even the most seasoned people can have mishaps. Treat hazard labels, SDS sheets, and safety drills as must-do, not just red tape. Keeping up with current safety guidelines from trusted bodies like NIOSH and OSHA makes sense. Learn from every near-miss—turn each mishap into a better habit. Encourage everyone in the lab to speak up if they spot unsafe behavior; peer accountability builds culture and keeps people on their toes.
The Bottom Line
Handling chemicals like 4-Butyl-3-fluorobiphenyl can be routine if you respect the risks and rely on training, equipment, and teamwork. Mistakes come from cutting corners. Wearing the right gear, working in ventilated spaces, and chasing down every splash or spill doesn’t just protect your health; it keeps the entire lab humming along without drama.
What 4-Butyl-3-fluorobiphenyl Means for Safety in the Lab
Working in chemistry labs, organic molecules often bring surprising risks. Even compounds that seem stable at first can react sharply when left in the wrong spot or exposed to light or air for too long. Plenty of folks skip over the boring parts of safety data sheets, but one thing keeps popping up: chemical stability hinges on smart storage. With 4-butyl-3-fluorobiphenyl, these rules matter as much as they do for any research chemical, and maybe more, since both the aromatic core and the fluorine atom can introduce stubborn reactivity if handled the wrong way.
A Cool, Dry Spot Wins Every Time
Over the years, small lapses in routine—say, leaving vials near a window—taught me that light and moisture turn “stable” compounds into cleanup headaches. 4-butyl-3-fluorobiphenyl responds best to the age-old rule: keep it in a cool, dry place. Temperatures around typical ambient room levels (15–25°C) work, but labs with high summer heat should lean toward refrigerated storage. Many labs designate flammable solvent cabinets for this sort of compound, since biphenyls fall on the list of flammable organic substances. Light can also affect aromatic rings or fluorinated groups, causing slow breakdown over time, though 4-butyl-3-fluorobiphenyl stays stable under normal storage if shielded from direct sunlight.
The Importance of Airtight Containers
Organic chemists know how sneaky air exposure can be. Screw caps sometimes aren’t tight enough, and humidity sneaks through. By using well-sealed glass vials with PTFE-lined caps, moisture and airborne contaminants stay out. Humid conditions encourage impurities or unwanted reactions, and even slow hydrolysis can build up trouble if the compound isn’t sealed right. Keeping containers upright, with accurate labeling and date of receipt, helps anyone using the stockpile know what they’re grabbing.
Personal Experience: Learning the Hard Way
In one of my earliest projects, I had a biphenyl derivative shelved close to a sink. A slow leak from the plumbing led to condensation and minor rust in the area; after a few months, some of the bottles had crusted impurities along the seals. Even though 4-butyl-3-fluorobiphenyl ranks lower on the “dangerous decomposition” scale compared to more reactive agents, repeated exposure to humidity and stray solvents left the sample questionable. That early oversight drilled home the habit of double-bagging chemicals and using desiccant packs in storage containers, especially if the local humidity can hit above 60%.
Solutions for Better Storage Practices
Small changes can cut down on accidents. Invest in airtight glass with real chemical resistance. Use secondary containment trays. Add silica gel or molecular sieves to cabinets holding organic compounds. Regularly check chemical stocks and dispose of old material. For those in shared spaces, establish basic rules for returning bottles and logging dates; good labeling means fewer mistakes later on.
For anyone shipping or transporting 4-butyl-3-fluorobiphenyl, consult all local rules. Use triple-packing and ensure that absorbent materials line the packing just in case. Even packages handled by couriers can get rough treatment, and chemicals do not belong in the back of a hot car.
Staying Professional and Safe
No one wants a lab accident or a ruined experiment because someone forgot to screw on a cap or left a sample in the wrong spot. The best work I’ve seen has always come from teams that take chemical storage seriously—no shortcuts, clear instructions, and a little bit of redundancy. It’s the part of science that rarely gets attention on posters, but keeps everything running safely and smoothly.
Why 4-Butyl-3-fluorobiphenyl Matters
Anyone digging into organic chemistry or specialty compounds might run across 4-Butyl-3-fluorobiphenyl. This compound sits at the intersection of academic curiosity and industrial research. Some folks look for it as a building block in the synthesis of advanced materials or in pharmaceutical research. For the niche user, knowing where to buy it ties into the broader context of chemical sourcing, safety, and compliance.
Getting Real About Purchasing Chemicals
It gets tricky when looking to buy 4-Butyl-3-fluorobiphenyl. The average consumer doesn’t see this on pharmacy shelves, amazon baskets, or DIY hobby shops—true for most specialty reagents. Most requests come from researchers or commercial labs. You won’t often find a casual shopper looking for biphenyl derivatives at the mall, either. Legitimate chemical suppliers stick to regulated channels.
Why Regulation Shapes Access
Experience in the lab teaches respect for regulation. The chemical industry—especially in parts of North America and Europe—keeps a close eye on who buys what. You’ll bump into supplier vetting, Know Your Customer (KYC) checks, and a steady flow of paperwork. There’s a reason: some compounds hold potential not just for innovation, but for misuse. Even a benign substance finds itself regulated if it shares a molecular backbone with something controversial or if its synthetic route can pivot toward illicit ends.
Who Sells It?
A handful of chemical suppliers stock 4-Butyl-3-fluorobiphenyl, mainly for industrial, research, or academic use. Specialized vendors like Sigma-Aldrich, TCI, Alfa Aesar, and a few niche suppliers sometimes carry it. They don’t just hand it out, though—proof of legitimate use and often institutional affiliation are required. Suppliers check that buyers meet safety and legal standards, often asking for licenses, proof of business, and explanations of intended use.
The Pitfalls of Alternative Channels
It’s tempting to turn to third-party websites, gray market vendors, or “import from abroad” listings. Doing so risks more than just a low-quality product. Skirting official channels raises the chances of running afoul of both safety standards and the law. Counterfeits, contamination, or legal trouble become real concerns. I’ve seen labs crippled for a month when a single inauthentic shipment disrupted testing.
Solutions and Best Practices
People who need to buy specialized chemicals do best by forming relationships with reputable suppliers. That often means reaching out directly, talking to a sales representative, and establishing credibility. Getting familiar with local regulations helps avoid headaches. In some countries, a permit might be required, and sometimes an import/export license for cross-border orders. Building a sourcing trail through recognized vendors keeps your research on a safe, legal footing.
Final Words for Safe Sourcing
Searching for rare chemicals like 4-Butyl-3-fluorobiphenyl links into bigger questions about transparency and responsibility in science. For anybody new to the world of chemical procurement, there’s value in checking vendor accreditations and seeking advice from experienced researchers. It’s not just about ticking boxes—it’s about keeping your work above board and safe for everyone. Staying on the right side of the rules ensures the supply chain supports progress, not problems.