4-Bromo-2-fluorobenzoic Acid: Insight and Commentary
Historical Development
Chemists traced benzoic acid derivatives back to the early days of synthetic organic chemistry. The push to alter benzoic acid, giving drug makers and material scientists new tools, really took off in the twentieth century. The addition of halogen groups—like bromine and fluorine—to benzoic acid became more common as analytical techniques improved and needs in the pharmaceutical world became bolder. The story of 4-Bromo-2-fluorobenzoic acid fits right into this timeline, surfacing through a mix of curiosity about substituted aromatics and the search for chemical building blocks that shake up conventional biological activity. The compound didn’t just spring from a textbook—it’s the byproduct of years treading from basic benzoic acid, exploring halogenation, and then fine-tuning reactions to favor unique substitution patterns.
Product Overview
Anyone who has spent time with benzoic acid derivatives will spot the significance of 4-Bromo-2-fluorobenzoic acid. This compound plays a structural role in labs focused on pharmaceuticals, agrochemistry, and sometimes specialty polymers. Researchers are drawn to it for the balance of reactivity and stability that halogen atoms give the ring. Its structure—benzoic acid’s base with bromine at the fourth and fluorine at the second position—proves useful for those forging new paths with molecular scaffolds, and its commercial value leans on demand from R&D teams who want to improve drug candidates or crop protection products.
Physical & Chemical Properties
Experience in the lab reveals the white to off-white powdery crystals of 4-Bromo-2-fluorobenzoic acid. The compound stands out for its relatively high melting point, often in the range of 150°C to 155°C, marking it as more stable than simpler halogenated benzoic acids. The bromine atom gives a larger molecular weight and increases lipophilicity—a feature not lost on anyone working with it in DMSO or DMF. Fluorine’s presence on the ring tightens the electron distribution, making this molecule both less reactive than expected under certain conditions and surprisingly tough against ordinary nucleophiles or oxidants. Solubility stays low in water, but adequate in polar aprotic solvents, showing its affinity for organic synthesis.
Technical Specifications & Labeling
Every chemical, especially one meant for laboratory or regulatory scrutiny, needs clear labeling. Chemists depend on accurate molecular weights (233.00 g/mol for this compound), verified CAS numbers (commonly 445-93-2), and clear batch information for reproducibility. Labeling addresses not just identity but handling—highlighting caution symbols, purity (often above 97%), and storage guidance (sealed container, cool and dry place, away from strong oxidants or bases). Some suppliers add NMR or HPLC assay data, which helps confirm both purity and the substitution pattern—a step crucial for those in regulated sectors.
Preparation Method
Organic synthesis students see reactions split by selectivity—especially halogenation. Synthesis of 4-Bromo-2-fluorobenzoic acid typically starts from 2-fluorobenzoic acid or a closely related precursor. The bromination, usually through electrophilic aromatic substitution, runs in the presence of a Lewis acid like FeBr3 or AlBr3. With the fluorine group in the ortho position, bromination targets the para position, reducing risk of polysubstitution. Some methods skip direct halogenation, opting for Suzuki or other cross-coupling chemistry, especially for complex synthesis or higher purity. Afterward, acidification and purification follow, with a recrystallization step ensuring purity suitable for downstream applications.
Chemical Reactions & Modifications
This compound acts as both a target and a substrate. The carboxylic acid can transform into acid chlorides or esters, useful for peptide coupling or forming prodrugs in medicinal chemistry. The fluorine resists displacement, keeping the molecule’s backbone sturdy through a range of reactions. Bromine offers a versatile handle for cross-coupling moves. Chemists carry out Suzuki, Heck, or Sonogashira couplings, using this group as the entry point for building more complex aromatic systems. Halide exchange, amidation, and esterification also find a place in synthetic routes. Each reaction reshapes the core, helping engineers and biochemists adapt the compound to unique applications.
Synonyms & Product Names
This compound rarely hides behind unfamiliar aliases in catalogues. You’ll meet it as 4-Bromo-2-fluorobenzoic acid, but it pops up too as Benzoic acid, 4-bromo-2-fluoro-, or even as 2-Fluoro-4-bromobenzoic acid in certain regions or supplier lists. Some researchers refer to its position-specific code names, especially those working with custom analogs for tissue receptor binding studies or library synthesis. Recognizing its synonyms helps avoid mix-ups, which anyone who's worked in a multi-vendor supply environment knows can be all too common.
Safety & Operational Standards
Chemicals with multiple halogens don’t ask for respect—they demand it. I’ve seen enough mishaps in shared academic fume hoods to know how important sound safety protocols are. Standard use calls for gloves, splash goggles, and lab coats. The MSDS typically flags potential for mild skin and eye irritation. Inhalation can irritate airways, so local exhaust or proper fume hoods make a real difference. Waste must go to halogenated organic waste streams, never the sink. Spills, though rarely dramatic, need immediate cleanup, as dried dust can stick around and complicate later work. Training on these procedures should always be hands-on, not just a stack of papers to sign.
Application Area
Medicinal chemists recognize this compound as a starter or intermediate in drug development. Halogen groups often shape how molecules fit into enzyme pockets, modulate metabolic resistance, and tweak solubility profiles. Across patent filings, 4-Bromo-2-fluorobenzoic acid shows up in scaffolds for nonsteroidal anti-inflammatory drugs (NSAIDs), oncology candidates, and central nervous system agents. On the agrochemical side, it sometimes appears in libraries for new herbicide or fungicide candidates. Material chemists test it, alongside similar acids, in the quest for new monomers and specialty polymers, thanks to its ability to transfer both rigidity and reactivity into finished products.
Research & Development
Research teams see 4-Bromo-2-fluorobenzoic acid as more than a catalog additive—it’s a launching pad. Custom synthetic routes often spin off from this molecule as teams hunt for new pharmaceuticals, crop protectants, or high-performance materials. It shows up in fragment-based drug design efforts, forming the aromatic heart for bioactive compound libraries. In my own work, the availability of quality lots has sped up medicinal chemistry projects, freeing us from days wasted re-validating starting materials. Patent databases show a rising trend since the early 2000s in derivatives and analogs, spotlighting just how central this backbone has become to competitive research worldwide.
Toxicity Research
Every halogenated compound brings toxicity questions. So far, toxicity testing on 4-Bromo-2-fluorobenzoic acid reveals limited acute effects, mainly related to skin or eye irritation and not systemic toxicity. Like many benzoic acid derivatives, metabolism tends to form less harmful products, but bromine and fluorine substitution both warrant caution until more detailed in vivo studies return. Chronic exposure studies remain sparse, which highlights a real gap—especially in pharmaceutical work. Companies and regulators benefit from deeper investigations, feeding into risk assessments for workplace safety and regulatory approval of products built on this framework.
Future Prospects
With rising interest in halogen substitution and the push for next-generation small molecule drugs, this compound doesn’t look to vanish from shelves. Contracts with pharma and agrochemical firms consistently mention the need for new, modifiable aryl acids. Advances in selective halogenation and cross-coupling will only boost its profile as a standard intermediate. Tighter safety standards and demand for green chemistry could drive innovation in cleaner synthesis routes as well. The intersection of regulatory, research, and commercial priorities promises to keep the story of 4-Bromo-2-fluorobenzoic acid in motion for years to come.
Understanding the Structure
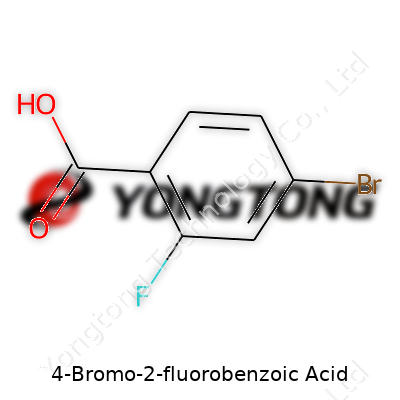
4-Bromo-2-fluorobenzoic acid shows up on lab shelves and textbooks, packed with utility and layered with detail. Anyone who has walked through a college organic chemistry lab has seen similar names scrawled on amber bottles, marked with hazard labels and, often, the distinctive acrid smell. The name tells most of its story for those willing to read it: a benzoic acid ring with specific substitutions—a bromine atom at the fourth position, a fluorine at the second.
Put together the basic backbone first. Benzoic acid carries the formula C7H6O2. Now, trade out a hydrogen on carbon two for fluorine, and a hydrogen on carbon four for bromine. The modifications don't simply alter the molecule’s shape. They signal a change in its reactivity, solubility, and even the kind of chemicals it likes to bond with or resist. The complete formula lands as C7H4BrFO2.
Why It Matters to Know This Formula
Digging into these changes, I remember using similar aromatic compounds during undergraduate research. Getting the substituted benzoic acid formula correct means the world when you have a shelf with close cousins—one swap in an atom, and the entire process heads down a different road. In academic and industry labs, accuracy in formulas avoids wasted hours, failed syntheses, or even dangerous reactions.
Bromine and fluorine bring heft and electronegativity to the molecule. This shifts the compound’s properties. In pharmaceuticals, small tweaks like this alter how drugs interact with targets inside the human body. Chemists need this formula not just to order the right sample, but to predict how the compound weaves its way through reactions—whether it gets broken down, activated, or neutralized.
Broader Impact and Responsible Use
Knowing the formula isn’t just about passing an exam or following a procedure. Each tweak in structure can affect how a chemical interacts with the environment, how easily it breaks down, and even its toxicity. Brominated compounds, for one, have faced scrutiny because of environmental persistence if misused or not disposed of correctly.
Manufacturers and labs have an ethical duty to handle, label, and dispose of chemicals like 4-bromo-2-fluorobenzoic acid with care. EPA and international regulations exist for a reason—these substances, while powerful tools, need respect. I’ve seen what happens when protocols slip: disposal fines, lab shutdowns, and sometimes harm to people not even involved in the original research. Vigilance here keeps researchers, the public, and the environment out of trouble.
Solutions and Best Practices
Standardized chemical databases—think PubChem or ChemSpider—save enormous time. These platforms vet their entries, so you don’t need to second-guess the chemical formula. Smart lab software links inventory to these trusted sources, flagging any swaps or mislabeling before accidents or wasted resources pile up.
Peer education plays into this as well. Mentoring students or new lab workers to double-check substitutions and reinforce the importance of correct formulas has real consequences, not just in safety but in quality of science. Academic labs train the next generation not only to see the formula as symbols, but as a gateway to understanding risk, effectiveness, and responsibility.
Getting the chemical formula for 4-bromo-2-fluorobenzoic acid right—C7H4BrFO2—means more than just passing a test. It shapes practices, safety, and the core of good science. This attention to detail keeps everyone moving forward and steers clear of preventable mistakes in chemical research.
Pharmaceutical Research and Drug Discovery
Most chemists come across 4-Bromo-2-fluorobenzoic acid while working on medicinal chemistry projects. This compound serves as a critical intermediate when building complicated pharmaceutical molecules. Its bromine and fluorine atoms encourage specific reactions, especially cross-coupling steps such as Suzuki or Heck reactions. These methods allow for new bonds that often can’t be achieved with simpler benzoic acids. In my previous role in a pharma lab, we often used this compound as a starting point for the rapid generation of diverse drug candidates. Even a small shift in a molecule’s substituents such as a bromine for a hydrogen helped tweak potency and safety. That’s not a small thing when you’re searching for a molecule that treats a disease with fewer side effects.
Agrochemical Development
Pesticide and herbicide research also draws heavily on specialized building blocks. 4-Bromo-2-fluorobenzoic acid helps scientists introduce key features into agrochemicals that manage pests while limiting environmental impact. Researchers often want to adjust molecules to break down safely or target only problem insects. By incorporating fluorine and bromine atoms in just the right spots, they’ve created new products that address upcoming regulatory standards. Talking to colleagues at an agrochemical firm reinforced this point—getting the right precursor often saves time in late-stage tests where regulatory requirements can derail years of work.
Building Blocks for Advanced Materials
Development of specialty polymers and liquid crystals involves a lot of trial and error. 4-Bromo-2-fluorobenzoic acid enters these labs as a useful fragment because its functional groups allow chemists to adjust transparency, flexibility, or responsiveness to light. I remember a collaboration with a team exploring organic light-emitting diodes (OLEDs) for display screens. Their engineering team required very precise positioning of atoms within their polymer chains. The benzoic acid segment with both bromine and fluorine was critical for tuning the emission properties. These adjustments shaped how the colors performed in a smartphone screen, all starting with the right building block at the right location in the chain.
Challenges in Handling and Safety
Lab safety and environmental impact remain on the minds of professionals who work with halogenated compounds. 4-Bromo-2-fluorobenzoic acid demands careful management—its halogen content means improper disposal pollutes water sources or harms wildlife. I’ve worked with green chemistry initiatives aimed at reclaiming or reducing use of hazardous building blocks. Scientists continue to search for methods that simplify synthesis with better yields and less waste. Anyone working in these fields recognizes the pressure from both inside and outside the industry to lower the risk footprint while preserving the unique benefits these chemical fragments bring.
Looking Forward
The reach of 4-Bromo-2-fluorobenzoic acid extends across pharma, agriculture, and materials science. Demand keeps rising for smart building blocks that speed up discovery and provide an edge in crowded markets. Factoring in sustainability, the chemical industry faces pressure to refine processes, invest in safer synthesis, and responsibly source or recycle these valuable compounds. Those who can balance innovation with responsibility are likely to shape the next wave of breakthroughs—from safer medicines to cleaner crops to brighter screens.
Why Practical Storage Matters More Than You Think
Anyone working around chemicals like 4-Bromo-2-fluorobenzoic acid knows that details matter. This is a solid chemical used in research labs and production lines, known for its role in pharmaceutical and organic synthesis. Improper storage can lead to wasted material, ruined experiments, and even safety hazards. Years in the lab taught me that the most interesting reactions tend to happen—unintentionally—when people cut corners with storage or ignore the little print on a safety sheet.
Temperature and Environment: Get It Right from the Start
Keeping this compound at room temperature might seem harmless. But temperature shifts in a typical storeroom during summer can push that “room temperature” into the danger zone. Stick to a cool, dry spot out of direct sunlight. Heat can not only mess with the purity but also speed up unwanted degradation. Humidity is the enemy here. Moisture in the air can turn a fine crystalline powder into a sticky mess. Once, I watched a full container clump up into a useless brick because a summer storm blew in and someone left the window open in storage. Use a desiccator or toss a few silica gel packs into the cabinet as backup.
Containers Make a Difference
Glass or high-density polyethylene containers with tight-fitting lids create a solid line of defense for most acids—this one included. Metal lids tend to rust fast around acids, and plastic bags don’t block enough moisture. Reusing old containers tempts fate, since residual chemicals or wild pH can trigger unpleasant surprises. A colleague once grabbed an unwashed jar, only to wreck their sample because last week’s experiment left traces of base behind.
Labeling: Not Just for Safety Inspectors
Clear, durable labels sound simple, but they’re often overlooked. Label with the full name, date, and hazard warnings. My own habit includes adding a color-coded dot for quick ID. That five extra seconds prevents confusion, especially when a dozen similar jars fill your shelf. In a shared workspace, missing labels breed chaos and lawsuits.
Personal Protection During Handling
Nitrile gloves keep skin safe from accidental splashes or spills. 4-Bromo-2-fluorobenzoic acid isn’t the nastiest thing on the shelf, but direct contact can still cause irritation. Splash goggles give your eyes a fighting chance in case of a slip—a lesson I learned the hard way while refilling a bottle. Lab coats stay standard, and always keep spills contained with bench pads or trays. Inhalation isn’t a top concern for solids, but working near good ventilation or a fume hood never hurts.
Disposal: Clean and Legal
Disposing of any organic acid means following local hazardous waste rules. Flushing chemicals down the drain once felt like a tradition, but that attitude creates long-term environmental headaches. Collect waste in a clearly labeled and sealed container, then contact an approved chemical disposal service. Check the safety data sheet for regulatory specifics; most suppliers provide them online in easy-to-access format.
Solutions For Better Practices
Simple steps pay off: train everyone who enters the lab, schedule monthly inspections of your storage space, and keep copies of safety data sheets in arm’s reach. Photos of your storage setup help during audits or after an emergency. Some labs rotate chemical stock, so older bottles get used first—nothing goes to waste, and nothing sticks around long enough to degrade.
The Value of Purity in Research Chemicals
Purity isn’t just a number stamped on a certificate; it’s a cornerstone of reliability for researchers. Handling 4-Bromo-2-fluorobenzoic acid in the lab, even a tiny dip in purity changes results. Picture a trial where a single contaminant leads to false readings, lost time, and wasted investment. High purity stands as a pledge that what goes into a reaction is exactly what’s meant to be there.
Measured Purity and Testing Methods
Purity of 4-Bromo-2-fluorobenzoic acid usually gets measured by high-performance liquid chromatography (HPLC) or gas chromatography (GC). Top-tier products routinely reach at least 98% purity. Quality certificates detail both the percentage and the test methods used. This kind of transparency helps chemists verify data on their end—cross-checking the supplier’s claims with their own findings builds trust that can’t be faked.
I’ve seen suppliers claim big numbers but change tune the moment you ask for a certificate. Any reputable supplier provides proper documentation quickly—showing real HPLC traces, batch numbers, and the date the material was tested. Once, I requested this from a new vendor, and their willingness to deliver every single detail convinced me I could trust their product for my work. It saves headaches and sets a solid foundation for results.
Why Purity Matters Beyond the Data Sheet
Small amounts of impurities in 4-Bromo-2-fluorobenzoic acid may seem minor, but in synthetic chemistry, they can change the path of a reaction. An impurity that’s chemically similar to the target compound slips into side reactions, confusing downstream analysis. In pharmaceutical development or materials science, this type of uncertainty can invalidate an entire research line.
Low purity doesn’t only spoil results; it risks safety. Uncharacterized contaminants in a compound can introduce unknown hazards. Labs often enforce strict acceptance criteria—98% as a baseline, with clear identification of remaining components. This level of detail supports safety assessments and keeps projects on track.
Ethics and Responsibility: Following E-E-A-T Principles
Suppliers and researchers both hold a responsibility to meet high standards. Experience shows, when supply chain partners cut corners, trust erodes quickly. At times, the cheaper source looks tempting, yet poor documentation or inconsistent testing quickly reveals the cracks. Experience, openness about sourcing, and providing up-to-date COAs are all part of earning trustworthiness.
Experts recommend buying only from established vendors with a traceable track record. Always push for official COAs that state actual numbers for purity, include analytical methods and detection thresholds, and mention analysts’ names. Responsible companies keep their testing equipment calibrated, use validated methods, and allow third-party audits when requested.
Improving the System, Step by Step
Education lifts quality across the board. Suppliers who invest in staff training and in equipment maintenance end up delivering better materials. Clear communication—being able to ask a supplier for an HPLC readout and actually get it—creates a feedback loop that pushes everyone higher. Problems still creep in sometimes; that’s where open reporting and swift recalls play a role.
Purity in 4-Bromo-2-fluorobenzoic acid isn’t just about numbers—it's about integrity, scientific rigor, and honest relationships throughout the chemical supply chain. Skilled researchers know how to check and verify, but finding partners who don’t cut corners makes every step smoother for both science and safety.
The Need for Quality and Trust in Chemicals
Anyone who has ever worked with fine chemicals knows just how important it is to trust what’s inside the container. Whether running research in a lab or producing batches in a bigger facility, everybody wants answers to basic questions. Is the substance pure? Does it have contaminants? Can I get a breakdown before I spend money or run a synthesis? The Certificate of Analysis, or COA, pulls the curtain back. For something like 4-Bromo-2-fluorobenzoic acid, a lot rides on it.
What’s in a COA, and Why Bother?
At its core, a COA lays out the facts. It usually covers the full chemical name, lot number, purity by HPLC or GC, and checks for heavy metals, solvents, and moisture. If you’ve ever hit a setback in a lab, you know what a lifesaver it is to spot an error early. Impurities, wrong melting points, or out-of-spec results can set back drug discovery, choke off data in material science, or even compromise safety. Without looking at a COA, it’s really a guessing game.
Can You Get a COA for 4-Bromo-2-fluorobenzoic Acid?
Reputable chemical suppliers will offer a COA for each batch of 4-Bromo-2-fluorobenzoic acid they sell. Buyers often forget this step, but skipping it means playing with fire. Chemical properties can shift from batch to batch; solvents linger or trace metals creep in from glassware or packing lines. It is not unusual for researchers to demand a recent COA before finalizing orders. Leading suppliers provide downloadable COAs linked to the lot, or email them on request—they know buyers watch these details.
The Role of Transparency and Accountability
A COA does more than satisfy bureaucratic requirements. It builds trust. Without transparency, researchers can’t rule out contamination, and mistakes go undetected. The US Pharmacopeia and ISO standards both agree: traceability is vital. If a problem crops up down the line, it’s possible to trace where it went wrong based on the information locked in a COA.
Earning Confidence in the Supply Chain
It gets tempting to cut corners, especially for rare intermediates and building blocks. But if you’re using a material like 4-Bromo-2-fluorobenzoic acid in medical research, you can’t risk getting it from unknown sources or sites that won’t show a COA. Counterfeit and substandard chemicals still find their way into the market. I’ve seen colleagues lose months of work to failed reactions, all because of tainted or mislabeled stock. Strong suppliers know their worth hinges on what their COA says.
Fixing the Gaps
The chemical industry keeps growing, and buyer expectations change. Labs and institutions can help by demanding COAs up front, storing them, and checking authenticity. Digital COAs reduce hassle and tie results to specific batches. Regular audits, supplier reviews, and proficiency checks also keep standards high. No process is perfect, but diligence pays off.
In Practice
Working chemists and lab managers should always ask for the latest COA with each shipment of 4-Bromo-2-fluorobenzoic acid. Review the numbers, scan for unusual values, and make sure everything matches paperwork. If suppliers can’t provide a COA, it’s a warning flag. Reliable labs keep COAs on file and encourage open sharing of QC data—in academic labs this means the difference between reproducible work and endless troubleshooting.