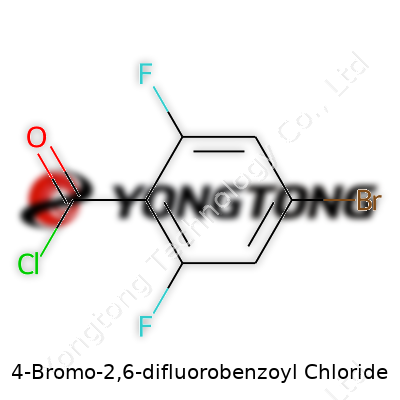
4-Bromo-2,6-difluorobenzoyl Chloride: A Detailed Exploration
Historical Development
Scientists started looking at halogenated benzoyl chlorides decades ago, recognizing that small tweaked molecules can become building blocks for breakthroughs across pharmaceuticals and advanced materials. Out of this group, researchers isolated 4-Bromo-2,6-difluorobenzoyl chloride, picking up on how the bromine and fluorine atoms on the aromatic ring open new paths for synthesis. Chemists in the late 20th century realized that putting both bromine and fluorine on a benzene ring dramatically shifted both reactivity and uses, pushing forward investigations into how to use these properties practically. Over time, this compound transitioned from being just another interesting bench chemical to a key player in specialty synthesis, especially as demand for more efficient reaction partners grew in research labs and manufacturing settings.
Product Overview
4-Bromo-2,6-difluorobenzoyl chloride doesn’t show up in everyday conversations, but labs rely on it as a foundational starting material. Chemically, it bears the classic structure of a benzoyl chloride but stands out because of two tightly held fluorine atoms and a single bromine atom arranged on the aromatic ring. This unique combination shifts its behavior compared to plainer counterparts and gives it utility wherever precise modification matters. Chemists value its reliability in controlled reactions as well as its role in assembling complex molecules for industries ranging from pharmaceuticals to crop protection.
Physical & Chemical Properties
Staring at a vial of this compound, you'd see a pale to off-white solid or sometimes a light yellow crystalline powder. It sits comfortably at room temperature, but don’t let that fool you—its benzoyl chloride backbone makes it pretty reactive, especially with things that like to grab onto acyl groups. Weighing in with a molecular weight around 269.45 g/mol, it dissolves best in common organic solvents like dichloromethane or chloroform, which makes sense given its hydrophobic structure. It resists decomposition under normal conditions, but at higher heats or in moisture-rich environments, you’ll see it hydrolyze into the corresponding acid.
Technical Specifications & Labeling
Bottles of 4-Bromo-2,6-difluorobenzoyl chloride must come with clear labeling: product name, batch number, purity (often above 98%), recommended storage conditions, and hazard information. Packaging helps keep it dry and away from sunlight, often in amber glass bottles or tightly sealed containers. Labs follow strict inventory controls to track its movement, and safety datasheets—summarizing risks for eyes, skin, and inhalation—stay close at hand. Technicians keep gloves, goggles, and chemical-resistant coats nearby, knowing that even brief exposure can irritate or sensitize.
Preparation Method
Making this compound usually starts with the right fluoro- and bromo-substituted benzoic acid. Once in hand, chemists react it with thionyl chloride or oxalyl chloride, transforming the carboxylic acid group into the reactive acid chloride. This doesn’t always come out clean, so experienced hands purify the end product by vacuum distillation or recrystallization, pulling off contaminants like unreacted acids or leftover reagents. The process hinges on careful temperature control and dry conditions. Even a little water throws things off, causing hydrolysis and knocking yield down.
Chemical Reactions & Modifications
This molecule serves as a reliable acylating agent. The electrophilic character introduced by benzoyl chloride’s carbonyl carbon, together with electron-withdrawing halogens, means it reacts swiftly with nucleophiles. Chemists tack it onto amines to make amides, hook it to alcohols for esters, and even use it in Friedel–Crafts acylation to create new aromatic ketones. The bromine atom, sitting at the para position, opens the door to cross-coupling reactions—Suzuki, Heck, or Stille—helping scientists build up larger, functionally diverse molecules. Meanwhile, those two fluorines increase stability, especially in hostile chemical environments, which matters quite a bit in material science or medicinal chemistry applications.
Synonyms & Product Names
There’s rarely just one way to refer to this chemical. In catalogs or published research, you may find it called 4-Bromo-2,6-difluorobenzoyl chloride, 4-Bromo-2,6-difluorobenzoic acid chloride, or its systematic name, benzoyl chloride, 4-bromo-2,6-difluoro-. Chemists also rely on identifiers like CAS number 885499-01-6 to eliminate confusion during procurement or inventory management, especially when similar-sounding compounds crowd the shelves.
Safety & Operational Standards
Handling this compound pulls in a suite of precautions. Its reactive chloride group releases hydrogen chloride fumes in contact with water or moist air, burning the throat and eyes. Gloves, eye protection, working fume hoods, and lab coats aren’t just recommendations—they’re non-negotiables. Storage demands dry, cool, and ventilated spaces, and chemists never leave the bottle open any longer than needed. Spill control kits with neutralizing agents and absorbent pads stand ready, since small lapses lead to costly interruptions or even injury. Training forms the backbone, with lab personnel drilled on procedures for exposure, waste disposal, and fire control.
Application Area
Industries making active pharmaceutical ingredients draw on 4-Bromo-2,6-difluorobenzoyl chloride for amide synthesis, where tightly controlled modification guarantees the right bioactivity. Agriculture companies use it to assemble new crop-protection chemicals, tapping into the bug-fighting power that halogenated aromatics can unleash. Materials scientists see value in its stability, folding it into functional polymers or specialty coatings to boost performance under harsh conditions. Even outside industrial scale, academic groups test its utility for pushing synthetic boundaries, piecing together complex targets for basic and applied research alike.
Research & Development
R&D teams keep pushing the envelope—using 4-Bromo-2,6-difluorobenzoyl chloride as a linchpin for reaction optimization, catalysis studies, and drug candidate assembly. Medicinal chemists rely on it for making new scaffolds that interact with disease targets more selectively and effectively, often exploring analogs to maximize efficacy and minimize toxicity. Some groups trial it in click chemistry or in polymer cross-linking, chasing after stronger or smarter materials. Trends show rising interest in combining this compound with new ligands or catalysts to boost yields, shorten reaction times, and slash costs.
Toxicity Research
Toxicologists test this compound for its acute and chronic effects. Its acid chloride group makes it a potent irritant, and studies confirm it damages mucous membranes, skin, and eyes upon direct contact. Animal models indicate potential respiratory harm if inhaled. Some metabolites from downstream chemistry—when not fully degraded or neutralized—pose additional risks if mishandled. Data from laboratory accidents highlight the need for strict procedural compliance; even low-level, repetitive exposures carry risks of allergic sensitization or more severe respiratory problems. Ongoing research focuses on clarifying safe exposure limits and improving workplace safeguards.
Future Prospects
Looking ahead, I expect 4-Bromo-2,6-difluorobenzoyl chloride to keep its foothold as a specialty reactant, especially as demand grows for next-generation pharmaceuticals, performance polymers, and efficient agrochemicals. Pressure mounts to develop greener manufacturing routes, so advances in flow chemistry and less hazardous chlorinating agents could reshape standard practice. Regulatory agencies pay closer attention to persistent chemicals in the environment, pushing for new disposal and containment methods. I see opportunities for improved selectivity using machine-learning-guided catalyst screening or process automation, reducing waste and cost. As the chemical industry evolves, this compound will stay relevant, provided chemists and engineers innovate around safety, scalability, and environmental stewardship.
Digging Into the Formula and Why It Matters
Anyone with experience in a chemistry lab understands that a compound’s chemical formula isn’t just a fancy string of letters and numbers. The formula C7H2BrClF2O for 4-Bromo-2,6-difluorobenzoyl chloride tells you how many atoms of each element come together to form the substance. In practical terms, that matters when you’re planning a reaction, managing safety, or checking regulatory boxes.
For students and researchers, one simple miscount can turn a promising synthesis into wasted hours—or risk a dangerous lab accident. I remember a graduate project where one researcher swapped a bromine atom for chlorine in a reaction plan, assuming reactivity and byproducts would follow the same trend. The difference nearly halted the project. This is why remembering such formulas isn’t about memorization for exams, but about understanding outcomes in the lab and in industry settings.
Behind the Elements in C7H2BrClF2O
Breaking down C7H2BrClF2O: Seven carbons, two hydrogens, one bromine, one chlorine, two fluorines, and one oxygen. Sure, it looks like a mouthful. But the mix isn’t accidental. The benzoyl chloride backbone finds use all over medicinal and agrochemical research, and those halogens—bromine, chlorine, and fluorine—shift how the molecule behaves. Allergenicity, reactivity, environmental behavior: these hinge on which atoms get tacked onto the benzene ring. A single change can mean the difference between a tool compound that leads to a new medicine and a chemical that sits on the shelf ignored.
Adding bromine and fluorine boosts the molecule’s electronegativity. Chloride on the benzoyl group turns it into an acyl chloride, upping its reactivity for further synthesis. Anyone trying to build custom compounds for a new drug target or next-generation pesticide values these changes since they control how and where the molecule reacts next.
The Stakes for Safety and Regulation
If you’ve spent time handling chemicals, the formula’s importance shows up in the safety cabinet too. Benzoyl chlorides don’t treat skin or lungs kindly. Regulations tail the use of any compound with multiple halogens. Governments take a hard look at production records and waste streams because mishandling can lead to toxic byproducts, like dioxins or halogenated solvents, left unchecked. Knowing the exact formula means following explicit protocols—ventilation, personal protective equipment, careful disposal. There’s no shortcut.
The Search for Better Approaches in Chemistry
Investment in green chemistry has started to pay off, with more labs looking for ways to replace hazardous reagents or reclaim halogen-rich waste. Some groups use microwave-assisted synthesis or flow chemistry to limit exposure times and cut down on byproducts from benzoyl chlorides. Chemists constantly develop methods that swap resource-heavy or toxic halogens for less persistent options. Sharing these findings openly—across academic, industrial, and regulatory fields—shortens the path from lab bench to safe, practical scale-up.
At its root, studying and understanding the formula C7H2BrClF2O brings you face-to-face with the reality of chemical research: precision, responsibility, and creativity matter. Memorizing a formula is just the start. Knowing what it means—and what can come next—keeps labs running safely and innovations moving forward.
Cutting Through the Complexity of Benzoyl Chlorides
Chemists look at compounds like 4-Bromo-2,6-difluorobenzoyl chloride and see a kind of Swiss Army knife for organic synthesis. This compound, with a handy mix of halogens and a reactive acyl chloride group, gives researchers a solid base for building more complex molecules. I remember sitting in a lab, the faint smell of acyl chlorides in the air, watching how a few drops could transform an entire reaction.
Key Role in Pharmaceutical Development
Pharmaceutical research leans on such compounds for creating new drug candidates. That bromo and the two fluorines on the benzene ring—there’s a reason they matter. Fluorine helps tweak the metabolic stability of a drug, making it last longer in the body. Bromine opens doors for further modifications, letting chemists attach new groups where needed. Drug discovery doesn’t always look glamorous, but it often depends on reactions with compounds like this, especially when developing treatments that require specific molecular shapes and resilience against breakdown.
A Building Block for Agrochemicals
The same properties that suit drug discovery also show up in agriculture. Chemical engineers use 4-Bromo-2,6-difluorobenzoyl chloride when searching for new insecticides or herbicides. If you’ve ever wondered why some crop protectants last through rain or sun, part of the answer comes down to how halogenated molecules hold up under environmental stress. This benzoyl chloride can react with amines and alcohols to produce structures that repel pests or boost plant health, which helps keep yields steady for farmers.
Creating Specialty Materials
Industries working with specialty polymers and high-performance coatings count on these reactive intermediates. I once visited a coatings plant that manufactured surfaces for medical devices and electronics. They used halogen-rich compounds like this one for creating barrier layers. The result: coatings that resist solvents or wear—something every phone manufacturer or hospital equipment supplier values. These materials don’t just appear by accident; their backbone often starts with fine chemicals that can be customized through stepwise addition of functional groups.
Research and Fine Chemical Synthesis
Academic chemists turn to this compound for methodological studies, including testing new catalytic reactions or designing new ligands for metal complexes. In the classroom and at the bench, these experiments push the boundaries of what’s possible with modern synthesis. A wider toolkit brings scientists closer to breakthroughs, and the ability to attach and remove chemical groups with precision remains essential.
Addressing Environmental and Safety Concerns
With great utility comes responsibility. The production and use of benzoyl chlorides, especially with halogens, can introduce hazards in the lab and the environment. Rigorous ventilation, safe waste handling, and reducing exposure make a difference; most stories of accidents begin with someone skipping a step. In recent years, green chemistry has become more present in discussions about synthesis, pushing for better ways to recycle or neutralize hazardous byproducts.
Innovation through Collaboration
Advances in chemical manufacturing don’t grow out of a vacuum. Partnerships between industry, academia, and regulators move the field forward—finding safer processes and new uses for existing compounds while keeping an eye on long-term sustainability. The work done with 4-Bromo-2,6-difluorobenzoyl chloride stands as one example of how small changes at the molecular level can drive big shifts in medicine, agriculture, and materials science.
Real Risks in a Real Lab
Anyone who's spent enough time in a research lab gets used to the idea that not all chemicals behave the same way. 4-Bromo-2,6-difluorobenzoyl chloride isn’t just another name on a label. It’s reactive, moisture-sensitive, and brings genuine hazards if someone gets careless. With an acid chloride group ready to react with water in the air, even minor exposure starts to break it down, releasing hydrogen chloride gas—bad news for both people and the shelf life of the bottle. I’ve seen what a leaking cap on a moist day can do: white fumes, a strong stinging odor, and wasted material that no one wants to handle twice.
Temperature and Containment
This compound belongs far from bright light and heat. My protocol at the bench relied on low temperatures – somewhere between two and eight degrees Celsius, usually a chemical fridge, away from food and drink. Warm storage slows reactions, but the biggest problem comes from swings in humidity. Any decent lab manager spends money on tightly sealed amber glass bottles. Not all plastics block out moisture, and acid chlorides eat through cheap screw caps surprisingly fast. I’ve seen labs reuse old bottles and get away with it for a few weeks, but the chemical smell always gives the game away. If you store it cold and dark, in containers with proven air-tight seals, stability goes way up, saving budgets and headaches.
Moisture Control
Desiccators—dry storage boxes—earn their space here. A jar filled with desiccant keeps the ambient air bone dry. Some chemists add a packet of silica gel inside the secondary container. Once, after transferring some solid aliquots, I wiped down a container and left a fingerprint of moisture. Next day, brownish stains marked where hydrolysis had started, turning expensive compound into an unusable goo. Scrubbing and double-sealing are all about buying time for sensitive reagents, especially ones this tricky.
Labeling and Emergency Prep
A sharp label gets plenty of attention. Hazard warnings, expiry dates, and open dates all help in the handover to the next researcher. I’ve seen accidents happen mostly when someone pulled a dusty bottle from the shelf, half-full and years past its best. Daily practice also involves spill kits stocked nearby, with sodium bicarbonate or similar neutralizers for acid spills. Fume hoods aren’t just a suggestion—ventilation becomes your frontline defense when less experienced chemists start uncapping bottles.
Waste and Degradation Problems
Old, decomposing batches turn sticky and lose reliability for synthesis. Some folks still try to push degraded material back into reactions. It only amplifies failures and messes with reproducibility. Proper inventory checks take five minutes—filter out suspect bottles quickly. Don’t dump them down the sink. Coordinating with chemical waste streams in your facility not only keeps the drains safe but saves someone from inhaling hydrochloric acid from an open trash bag.
What Works in the Real World
Suppliers sometimes arm users with fancy guidelines, but they all fall back on the same core idea: keep it sealed, dry, and cold. Every research group runs tight margins, so training newcomers on the right way to store these materials is just as practical as running purification columns. Small tweaks can lock hazard and cost in place, and keep work predictable—not just safer, but smarter in the long run.
Understanding Purity in the Lab and Beyond
Every time I check the certificate of analysis for a specialty chemical like 4-Bromo-2,6-difluorobenzoyl chloride, I think back to spilling a reaction in lab because of an unexpected impurity hiding in what I thought was a "clean" bottle. It took weeks to troubleshoot, and plenty of time staring at spectra. Purity matters not because of textbook reasons, but because even a little contamination can mess up yield, send an R&D project off course, or even put health at risk for colleagues down the hall.
Packed With Precision: How Purity Is Rated
On the market, most suppliers offer this compound at a purity above 97%. Analytical methods like HPLC and NMR back up those numbers. Some catalogs advertise “98%” or better. A well-known global supplier sometimes lists 99% for their “high purity” lab stock, and from the technical data they provide, those lots show almost nothing extra hiding in the peaks. At this level, traces of related benzoyl derivatives or residual solvents do pop up sometimes, but shouldn’t take the main stage.
From what I’ve seen, this molecule often appears as a white to off-white solid; anything else signals trouble. Discoloration or a strong odor sometimes mean decomposition—a sour memory from the time I opened a bottle straight from customs that lingered with the harsh scent of acid chlorides gone off. This is not just a cosmetic warning. Contaminants can kick-start unwanted side reactions, especially if you’re working on a tight synthetic sequence.
Why Higher Purity Costs and Pays Off
The price often jumps with each decimal of purity. Purifying halogenated, multifunctional benzoyl chlorides takes careful distillation or column work. Process engineers use advanced techniques to lock down side products—this labor and material cost reflects in the sticker price. My lab learned the hard way that “cheaper” lots worked fine for proof-of-concept, but left us cleaning up strange byproducts at scale. High purity saves that pain in pharma and crop science, where even trace contaminants can show up in regulatory filings or trigger batch failures.
Looking Beyond the Label
Before signing off on a purchase, I always request the vendor’s most recent batch COA and method of analysis. Just last year, an unfamiliar overseas supplier sent a shipment with fewer details, which set off alarms. Trust builds on transparency. Ask for spectra. Check the water and acid content, as acid chlorides love to react with traces of moisture, spawning HCl gas and side reactions that no glove box really stops. The trend now leans toward tighter specs, since clients in biotech and materials science push for “known unknowns” like unlisted impurities.
Finding Solutions for Sourcing
Sometimes the answer to high-purity needs isn’t just a bigger budget. I’ve contacted custom purification shops directly, especially if a project needs the cleanest material possible for sensitive downstream steps. Scaling up a reaction in a regulatory environment pushes for even more rigorous quality studies, sometimes including GC-MS impurity profiling. Sourcing partners who respond quickly to transparency requests and offer data up front win more trust, even if their product costs a little more on paper.
For any chemist or procurement officer, it pays to keep purity front and center. It’s not just about keeping tidy paperwork. It’s about keeping research running smoothly, projects truly moving forward, and everyone in the lab safe from surprise headaches.
Understanding What You’re Dealing With
4-Bromo-2,6-difluorobenzoyl chloride doesn’t pop up in everyday conversation, not unless you spend your days in a chemical lab. This chemical sits in that category where a little ignorance can cause big headaches. It’s used in organic synthesis, often as a building block to create more complex molecules. With a name this long, many forget how tricky it can be if not treated with the respect it demands.
The Hazards Around the Corner
Get a whiff of this compound and your respiratory system tells you things have gone wrong. 4-Bromo-2,6-difluorobenzoyl chloride isn’t just annoying to smell—it’s an acyl chloride, which reacts fiercely with moisture in your airways, potentially releasing corrosive fumes like hydrogen chloride and acid mists. Eyes sting, skin burns, and you quickly learn it’s not something to rush through. Splashes don’t just leave you with discomfort—they can do serious lasting damage. Skin gets red, blistered, and eyes might feel like they’ve been scraped. On top of that, it vaporizes rather easily at room temperature, so even careful handling means you’re never entirely out of the woods.
Safety Precautions: Everyday Habits, Not Just Protocol
I always double up on PPE around benzoyl chlorides. This isn’t just about ticking a box on a safety sheet. Lab coat, chemical-resistant gloves (nitrile does the trick), good goggles, and a face shield. Trying to convince yourself it’s overkill just doesn’t make sense. Fume hoods make a huge difference. Vapors linger, and even if you don’t see the immediate after-effects, repeated low-level exposure is playing with your long-term health. Fume hoods catch the nasty stuff before you breathe it in.
Spill something — don’t just rush for the paper towels. Keep spill kits close, and use an absorbent material made for acids and solvents. Scoop the mess—not your hand—onto the absorbent, and bag it up for hazardous waste disposal. Don’t even think about using water. Water makes it splatter and releases acid fumes. Think dry, controlled, and calm. And don’t ever eat, drink, or touch your face in the lab. Sounds simple, but easy to forget until something goes wrong.
What to Do if Things Go Wrong
Despite our best intentions, mistakes slip through. Skin contact calls for plenty of running water for at least 15 minutes. Eye wash stations exist for exactly these moments—use them, and don’t rely on a splash from a water bottle. If someone breathes this chemical in, get into fresh air and seek medical attention fast. Every chemist I know keeps emergency numbers right next to the hood because seconds count if someone reacts badly.
Building a Safety Culture
Sitting through chemical safety training makes more sense once you’ve handled compounds like 4-Bromo-2,6-difluorobenzoyl chloride. Peer accountability helps, too. I’ve caught friends prepping these chemicals without face protection, and it never feels annoying to remind someone. Fact is, nobody leaves home expecting a trip to the emergency room. There’s real value in rehearsing potential spills and exposure scenarios—not just reporting it after the fact. Awareness, proper equipment, and fallback plans make a real difference in the way you tackle volatile materials like this.
Safer Alternatives and Smarter Choices
Some newer labs are finding ways to use alternate reagents that do the same job with less hazard, or engineering reaction setups to automate addition and limit direct handling. Green chemistry pushes for less hazardous starting materials, and that’s worth pushing for, especially in educational settings where newer students might make more mistakes. Until then, respect the risk, keep up solid safety habits, and don’t cut corners. The science is important, but so is going home in one piece.