4-Bromo-2,6-difluorobenzoic Acid: A Deep Dive from Lab Bench to Modern Applications
Historical Development
4-Bromo-2,6-difluorobenzoic acid didn't just appear on the shelves of chemical suppliers overnight. Its story grows from decades of research focused on modifying aromatic acids for specific industrial and pharmaceutical needs. Years ago, synthetic chemists realized that benzoic acids set a solid platform for building more complex molecules. By the late 20th century, the value of introducing halogen and fluorine atoms became clear as demand increased for compounds showing both stability and reactivity. Research in academic and industrial labs led to bromination and fluorination protocols that are more selective and less hazardous than earlier attempts, laying the foundation for the syntheses used today. This compound’s place in targeted molecular design has only grown as organic chemistry techniques advanced and more industries recognized the need for functionalized benzoic acids.
Product Overview
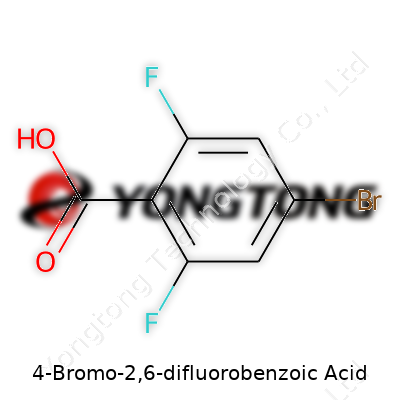
Anyone who has handled 4-Bromo-2,6-difluorobenzoic acid knows it stands out among benzoic acid derivatives. The combination of two fluorine atoms at the 2 and 6 positions and a bromine atom at the 4 position brings unique reactivity and a suite of downstream uses. Formulators often look for this compound when seeking a building block for agrochemical, pharmaceutical, or materials applications. The structure serves as a starting point for more elaborate molecules where regulated reactivity is needed. Even before reaching research labs, this acid goes through rigorous purification to remove unwanted isomers or side products, ensuring researchers and manufacturers can trust every batch.
Physical & Chemical Properties
4-Bromo-2,6-difluorobenzoic acid typically appears as a white to off-white crystalline powder. The high electronegativity of its fluorine atoms and polar carboxylic group combine to yield a melting point often above 150°C. The compound is sparingly soluble in cold water but shows better solubility in polar organic solvents like dimethylformamide and acetonitrile. Its molar mass sits comfortably under 250 g/mol, and the molecule displays both stability and strength due to the halogen atoms. Chemists recognize its acidity (pKa between 2 and 4) as a lever for further derivatizations. The electron-withdrawing groups tune both reactivity and physicochemical traits, such as partition coefficients and crystal structure, giving it a reliable profile for a wide range of downstream chemical reactions.
Technical Specifications & Labeling
Every reputable supplier knows that labeling and documentation can separate high-quality chemical products from the rest. For 4-Bromo-2,6-difluorobenzoic acid, typical labels outline chemical purity (≥98% for most applications), batch number, and precise molecular weight. Certificates of analysis usually feature detailed data such as residual solvent content, moisture levels, and possible heavy metal contamination. Packaging commonly prioritizes protection from light and moisture, using amber glass bottles or tightly sealed polyethylene containers. Storage recommendations usually point toward cool, dry environments away from sources of ignition and direct sunlight. Proper labeling proves critical, not just for safe handling but also for regulatory compliance if the compound enters sensitive applications such as drug synthesis.
Preparation Method
Preparative methods for 4-Bromo-2,6-difluorobenzoic acid tend to follow robust protocols refined over years of lab work. Synthesis often starts with 2,6-difluorobenzoic acid as the core. Chemists employ electrophilic aromatic substitution to introduce the bromine atom at the 4 position. Conditions might involve bromine or N-bromosuccinimide in polar aprotic solvents under controlled temperatures to avoid over-bromination or unwanted side products. Recent advances have led to greener approaches, such as using solid-state catalysts or less toxic brominating agents to minimize environmental impact. These efforts produce higher yields and cleaner reactions, which matters when scaling up from grams in the lab to kilograms in industrial settings. Post-reaction, purification often includes recrystallization or chromatographic techniques to ensure a high-purity end product.
Chemical Reactions & Modifications
Chemists frequently use 4-Bromo-2,6-difluorobenzoic acid as a substrate in cross-coupling reactions like Suzuki, Stille, and Negishi protocols. The bromine at the 4-position reacts smoothly with organoboron or organotin reagents in the presence of palladium catalysts, paving the way for tailored substitution with aryl, alkyl, or vinyl groups. The acid group serves as an anchor for amidation, esterification, or even reduction to alcohols. Both fluorine atoms act as shields, modifying electron density and controlling how the ring reacts with nucleophiles or electrophiles. These options make the compound highly attractive to medicinal chemists looking to fine-tune activity or physical properties in a new lead series. Such versatility keeps it in demand for both exploratory research and scale-up into commercial syntheses.
Synonyms & Product Names
The complexity of international trade and research calls for clear nomenclature, and 4-Bromo-2,6-difluorobenzoic acid goes by several names. Some refer to it as Benzoic acid, 4-bromo-2,6-difluoro-, or 2,6-difluoro-4-bromobenzoic acid. Catalogs might list it under different registry numbers or abbreviations, usually based on IUPAC rules. Alternate product codes arise, depending on manufacturer, but the structural diagram always stays the same. Confusion can arise from older literature that gives outmoded synonyms, so cross-checking with current databases keeps projects on track and mitigates ordering errors.
Safety & Operational Standards
Handling chemicals like 4-Bromo-2,6-difluorobenzoic acid isn’t just about understanding reactivity but also about personal and environmental safety. Because of the bromine and fluorine content, direct exposure can irritate skin, eyes, or respiratory tract. I always opt for gloves, lab coats, and splash-resistant goggles in the lab. Ventilation and proper fume extraction stop harmful vapors from building up. Industry standards emphasize safe storage — away from strong bases and oxidizers — and recommend clear labeling for spill response. Waste management policies call for segregating halogenated organic chemicals for specialized disposal. Adhering to regulatory standards, such as REACH in Europe and TSCA in the US, is a matter of legal responsibility as much as it is about protecting people and the environment.
Application Area
Anyone tracking emerging research in organic chemistry, material science, and drug discovery knows this compound finds a home in several fields. Medicinal chemists leverage its stable aromatic scaffold with modifiable groups for synthesizing kinase inhibitors, antiviral agents, or imaging probes. Materials scientists build on its ability to serve as a precursor for liquid crystals and specialty polymers, owing to its controlled symmetry and rigidity. Crop science teams explore its use as a lead compound for new agrochemical candidates — especially herbicides and antifungal agents — exploiting unique halogen combinations that enhance biological activity and persistence. Research demand often trails market developments, and every bump in interest reflects fresh opportunities for practical breakthroughs.
Research & Development
Much of the innovation related to 4-Bromo-2,6-difluorobenzoic acid comes from creative use in modern medicinal chemistry. For example, researchers often introduce this compound as a point of diversification in combinatorial chemistry libraries. Its substituents deliver beneficial steric and electronic characteristics that make it easier to achieve binding at complex biological targets. Beyond pharmaceuticals, R&D in electronics focuses on its derivatives for semiconducting materials. The acid group delivers tunable solubility, while the ring’s halogenation creates the right balance of conductivity and stability for organic thin-film transistors (OTFTs). Environmental scientists want new derivatives for sensors and selective absorbents. Each year, more publications reference its derivatives, evidencing a growing reputation in multiple research communities.
Toxicity Research
Concerns for safety push toxicological studies to the front of any conversation about benzoic acid derivatives. While toxicity data for 4-Bromo-2,6-difluorobenzoic acid still has gaps, analogues with similar halogen content give baseline expectations. Repeated exposure, especially to dust or vapors, often triggers respiratory or dermal irritation. Environmental persistence may be elevated due to multiple halogen atoms, so waste controls and monitoring sit on regulatory radars. Research in animal models aims to reveal more about metabolites, organ system impact, and any potential bioaccumulation. The industry pushes for more data to meet evolving global safety standards, especially if the compound moves toward larger-scale commercial roles. The responsible approach means pushing for more structure-activity data and environmentally friendly alternatives wherever feasible.
Future Prospects
Looking ahead, 4-Bromo-2,6-difluorobenzoic acid stands ready to play a bigger part in both new materials and drug leads. As machine learning models gain traction in drug discovery, compounds featuring unique substitution patterns like this one get more attention for screening campaigns. Sustainability pressures may spur researchers to update old synthesis routes, reducing solvent waste and energy use. Custom derivatives based on this scaffold may show up in advanced sensor technologies, organic electronics, and greener crop protection products. Ongoing research will guide next steps, but right now, demand remains steady from those developing molecular libraries and innovation-focused start-ups. The compound's role grows as science continues to chase after safer, more efficient, and more selective chemical tools.
Getting Under the Surface
Walk into any chemistry lab, and you’ll spot more than just glassware and gloved hands. What really pulls everything together? The strange strings of characters on bottles—those chemical formulas. Take 4-Bromo-2,6-difluorobenzoic acid, for example. The chemical formula for this compound is C7H3BrF2O2. This formula gives clues not just for making the compound, but for understanding its potential and its risks.
Why the Formula Matters
It’s easy to brush off chemical names as jargon, but accuracy in these details can affect everything from lab safety to pharmaceutical research. With 4-Bromo-2,6-difluorobenzoic acid, every atom tells a story. The molecule holds seven carbon atoms, three hydrogen atoms, one bromine atom, two fluorine atoms, and two oxygen atoms. Those numbers may seem small, but they set the boundaries for how the molecule reacts and interacts with its environment.
Fluorine and bromine, for instance, aren’t added just for convenience. They give the compound very particular chemical strengths—they change the acidity, the reactivity, and even the melting point. In my early research days, one wrong atom swapped during a synthesis meant failed reactions and wasted weeks. Asking a supplier for “the benzoic acid with bromine and two fluorines” isn’t enough. Without the precise formula, mistakes and confusion creep in fast.
Safety and Real-World Impact
The formula isn’t just trivia for chemists. It plays a big part in safety planning. Brominated and fluorinated compounds often find use in pharmaceuticals, materials science, and agrochemicals, but each atom affects toxicity, environmental persistence, and disposal requirements. In one municipal lab job, I saw how the absence of proper chemical documentation made for some dangerous guesses. We depend on formulas like C7H3BrF2O2 to track and dispose of high-risk substances safely.
Regulatory bodies base hazard labeling and storage instructions on formulas. For instance, the presence of bromine raises flags for many health and environmental regulators. Fluorinated compounds tend to be stubborn in the environment—that persistent, non-degrading property gets attention from EPA assessments worldwide. Supplies with precise labeling—formula and all—reduce costly errors and support both legal compliance and personal safety.
Supporting Better Practices
If you’ve spent time dealing with chemical suppliers or managing lab inventories, you know how details like C7H3BrF2O2 keep the wheels turning smoothly. Databases like PubChem or ChemSpider, which cross-reference formulas with hazard classifications and industrial uses, show their value every time someone looks up toxicology or handling information. Sharing accurate formulas isn’t about gatekeeping science; it’s about leveling the playing field so everyone—from high school science students to PhDs—has a common language.
There’s room to streamline this system. Adopting universal digital identifiers connected to chemical formulas helps. Stronger digital standards for labeling and safe data-sharing push the industry forward, cutting down confusion and accidents. As research and regulation keep changing, precision never goes out of style. In my experience, double-checking the chemical formula—that little string on the label—often saves trouble later on. That habit stays long after leaving the lab.
Behind the Chemistry
4-Bromo-2,6-difluorobenzoic acid lands in a category chemists call functionalized aromatic acids. That alone doesn’t tell you much. Start looking closer and you'll realize it brings two things to the table: a bromine atom and two fluorine atoms on a benzoic acid core. Anyone who has spent time in a synthetic lab knows these kinds of tweaks change the game for pharmaceutical and materials development.
Pharmaceutical Research Loves Precision
Medicinal chemists have a lot of respect for building blocks that let them play with structure. This compound offers up a mix of reactivity from the bromine and fluorines, making it a star when chemists want to build molecules that interact with living systems. Fluorinated groups often improve stability of drugs inside the body and can modify how a molecule fits into a protein. The bromine acts like a handle, letting researchers attach new groups in specific spots on the benzene ring. These tools come in handy for creating new antibiotics, cancer therapies, and even diagnostic agents. Drug discovery is no simple job; the right starting material saves months of trial and error.
Crop Protection Chemicals Keep Fields Productive
Fighting plant diseases and pests takes chemistry with guts. Agrochemical companies turn to molecules like 4-Bromo-2,6-difluorobenzoic acid to invent new herbicides and fungicides. The halogen atoms help create selectivity, so chemicals hit weeds or fungus without smothering crops. Farmers see direct results: healthier fields, less crop loss, and more food on the table. Developing new farm chemicals isn’t about fighting nature blindly—chemists look for compounds that break down safely. Using a benzoic acid core helps, since it sits at a crossroads of effectiveness and break-down rates, based on how chemists shape the molecule down the line.
Materials Science Builds Better Gadgets
Modern gadgets ask for better plastics, coatings, and specialty materials. Labs supply these thanks to finely tuned chemicals like 4-Bromo-2,6-difluorobenzoic acid. The bromine lets manufacturers bolt on tailored groups, turning a plain sheet of polymer into something water-resistant or bacteria-fighting. The fluorines help improve how materials hold up to heat, friction, or even exposure to harsh cleaning agents. As supply chains demand ever-more-specific products, the foundation starts with precision chemicals. Without access to these special ingredients, today's batteries, touchscreens, or protective fabric coatings would fall short.
Environmental and Human Health Matters
The strengths that make this compound valuable in labs can also pose risks if mishandled. It pays to stay current with best practices for personal safety and disposal. Regulatory agencies monitor how these building blocks move through the ecosystem and what happens after use. Local and global oversight matters: responsible sourcing, green chemistry principles, and proper waste management all count here. Investing in safe handling and greener processes isn’t just regulatory—companies risk reputation damage and long-term cost if they cut corners.
Finding Better Ways Forward
Industries have started to look for sustainable routes to make and use functionalized benzoic acids. Chemists designing new routes keep yields high and waste low. Some places experiment with biobased feedstocks, which cuts down on fossil fuel dependence. Real breakthroughs often come from collaborations between government, university, and private labs. People in science, business, and policy will make bigger gains when honest conversations about safety, sustainability, and open access happen often. The future for specialty chemicals like this one looks promising—if we keep our eyes on both progress and responsibility.
Understanding the Stakes
The quest for high purity in chemicals such as 4-Bromo-2,6-difluorobenzoic acid isn’t just a checkbox for lab protocols. It shapes the reliability of experiments, the accuracy of results, and the safety implications across industries. Over the years, working in different research labs, I’ve seen talented chemists frustrated by inconsistencies in their data—all traced back to lower-grade reagents. Precision in purity becomes more than a technical demand; it's about integrity in science.
Manufacturers and vendors tend to specify a minimum purity, often around 98% or higher for research-grade material. Analytical standards push this even further, usually requiring 99% minimum purity. Trace impurities, such as other halogenated benzoic acids or left-over solvents, can sabotage analytical methods, especially when running sensitive detection work like LC-MS or NMR. Purity testing follows a combination of melting point analysis, HPLC, and NMR to spot both organic and inorganic traces.
Why Laboratories Care
Even small amounts of residual bromide, fluoride, or unreacted precursor will skew spectral data. In pharmaceutical research where 4-Bromo-2,6-difluorobenzoic acid might be a building block, contamination risks end up costly. Some colleagues learned this the hard way; one analytical run with a batch at 97% purity led them on a wild goose chase, chasing signals that disappeared with truly pure material. Mistakes like that waste resources and patience.
In regulated environments, such as those following cGMP or GLP, specifications aren't negotiable. A standard SDS from a reputable supplier typically details the purity, states residual solvent content (like dichloromethane or methanol), and sets maximum allowable levels—often, solvents get held to under 0.5%. Control over metal impurities becomes even tighter. No matter the discipline, consistent purity means scientists spend less time troubleshooting and more time innovating.
What’s in a Specification?
A trustworthy specification sheet for this compound doesn’t just mention “purity ≥98%.” It includes identity confirmation by melting point and NMR, limits on water content (often under 0.5%), and caps on specific trace contaminants. For anyone handling synthesis, this transparency is key. When a certificate of analysis breaks out the impurity profile rather than lumping all “unknowns” into a single, vague percentage, confidence climbs. Nobody wants to guess about what’s hitchhiking in their flask.
Some of the best results I got followed a direct conversation with suppliers. Asking about their batch-to-batch consistency, how frequently they verify purity, and access to actual chromatograms made all the difference. Open exchange stands above flashy datasheets. Working this way, I’ve avoided costly repeat syntheses and sidestepped confusion in interpreting data.
A Path Forward: Solutions and Responsibility
Tighter collaboration between suppliers and end-users would cut down many headaches. If labs demand clear, batch-specific purity data, suppliers feel the pressure to improve standards. On the user side, nothing beats routine incoming quality checks: run your own HPLC or NMR on every new bottle, regardless of trusted reputation.
Ultimately, purity isn’t just a number. It signals the commitment of everyone involved, from manufacture to bench scientist. Serious research starts with serious ingredients—anything less, and we gamble with the truth our data holds.
Why Storage Choices for Chemicals Matter
Working in labs over the years has taught me that getting storage right can shape the quality and safety of the whole workspace. No bench researcher wants a headache from leaking bottles or surprises from unstable compounds. 4-Bromo-2,6-difluorobenzoic acid may sound obscure, but it lands in many reactions, especially if you’re digging into pharmaceutical or materials research. Keeping it stable and safe isn’t complicated, but there’s no room for guesswork.
Temperature and Environment
Many organic acids settle down best at room temperature — and from experience, stable room temperature trumps setups that fluctuate wildly. I’ve seen the impact of placing reactive chemicals near lab windows or radiators; spoilage can become a quiet problem there. For this acid, aim for a cool, dry spot, away from sunlight or any direct source of heat. Humidity sneaks into bottles, clumps powder, and can trigger slow reactions that erode sample purity. My advice: Use desiccators or dry cabinets if you have them, or the driest cupboard on site.
Sealing and Containers
No chemical lasts long in a flimsy or poorly sealed container. Glass makes the safest bet for acids like 4-Bromo-2,6-difluorobenzoic acid, especially with those famous PTFE-lined caps. After watching plastics warp and degrade, I’d say don’t risk storing even small amounts in soft plastic. After each use, screw the lid down tight. Wipe threads to prevent crusty buildup — those crystalline rings always show where moisture has crept in.
Avoiding Cross-Contamination
In shared labs, it’s easy to grab an unlabeled bottle or lose track of which sample’s which. This acid has bromine and fluorine atoms — so I treat it like I would any compound with reactive halogens: keep it away from strong bases, oxidizers, or anything that could spark a reaction. Segregate acids on their own shelf, and store it upright so caps never wick spilled liquid.
Labeling for Transparency
It sounds basic, yet I’ve seen far too many bottles with fading ink or sticky notes as labels. Use waterproof markers or printed labels that list the compound name, concentration, hazard info, and the date received or opened. If the bottle ever moves between workstations, tracking the age and history helps spot spoilage early.
Ventilation and Spill Planning
Vapors from halogenated acids aren’t pleasant. I always check that the storage area has good airflow. Emergency spill kits — absorbent pads, neutralizing powders — should sit close by, not buried under stacks of paperwork. After seeing a careless spill eat through a benchtop, I make sure new students know exactly where to find cleanup gear.
Responsible Chemical Management
Inventory matters. Keep a record of quantities, location, and annual checks. Some regulations call for this already, but honest tracking avoids expired or degraded stock, which only gets riskier as time passes. Disposal through proper channels beats flushing down a sink and risking wider harm.
Building Habits That Stick
Anyone working with chemicals owes their team — and themselves — clear habits. Even for a compound as specialized as 4-Bromo-2,6-difluorobenzoic acid, good storage keeps research on track and lets everyone focus on science, not the fallout from forgotten best practices.
Getting Familiar With the Chemical
Most people have never heard of 4-Bromo-2,6-difluorobenzoic acid unless they’re deep into chemistry labs or dabbling in pharmaceutical work. This compound isn’t found at your local pharmacy or hardware store. Back in college, running reactions with halogenated aromatics required gloves, eye protection, and some real respect for the bottles you handled. This acid has a bromine atom along with two fluorine atoms stuck to a benzene ring, and that makes the molecule worth some extra caution.
Hazards: What Does the Evidence Say?
I’ve handled enough chemicals to know that halogenated benzoic acids don’t just blend into the background. 4-Bromo-2,6-difluorobenzoic acid isn’t on the list of the world’s most hazardous chemicals, but the bromine and fluorine put it miles away from ordinary vinegar or citric acid. Brominated and fluorinated organics can cause skin irritation, eye injury, and respiratory problems if inhaled as dust. I’ve seen folks brush off the MSDS (Material Safety Data Sheet) for less complicated acids, then come away with red hands or watery eyes.
The available safety data from Sigma-Aldrich and chemical suppliers lists common risks: skin and eye irritation, possible respiratory irritation, and environmental toxicity due to bioaccumulation. Even though it doesn’t explode or catch fire easily, it deserves more respect than simple household acids. The Environmental Protection Agency (EPA) classifies brominated organics as pollutants, and there’s good reason. These molecules can build up in soil and water, and once they’re there, removing them isn’t simple.
Handling Precautions That Matter
People who regularly work with this chemical will always put on gloves, a lab coat, and safety goggles—no exceptions. I’ve personally experienced how a small splash, even from a tiny amount of a related brominated acid, can make skin feel like it’s been sunburned. Fume hoods do more than look high-tech—they’re essential because this compound can irritate the lungs if it gets airborne. It’s not something to measure out on your kitchen counter.
In a good lab, 4-Bromo-2,6-difluorobenzoic acid waste never winds up in the regular trash or the drain. Instead, it goes into hazardous waste containers, making disposal expensive and careful. If a spill happens, it’s not a job for paper towels and tap water; dedicated spill kits, absorbents, and sometimes even professional cleanup become necessary.
Taking Responsibility With Chemicals
Downplaying the hazards of specialty chemicals leads to dangerous habits. I’ve watched co-workers get lackadaisical only to face surprise allergies and persistent coughs. Big names in chemical distribution, including Fisher Scientific and Sigma-Aldrich, highlight the need for controlled storage—containers stay tightly closed, stored away from acids or bases that could cause a violent reaction.
Training and lab protocols aren’t just red tape, especially for compounds like this one. Regular hazard reviews, hands-on safety drills, and easy access to updated safety data sheets protect everyone in the room. There’s real peace of mind in knowing someone can handle a spill or accidental exposure properly. In an era when environmental and personal health carry real weight, treating 4-Bromo-2,6-difluorobenzoic acid with care isn’t just responsible lab practice—it’s common sense backed by experience, science, and the basic desire to keep everyone safe.