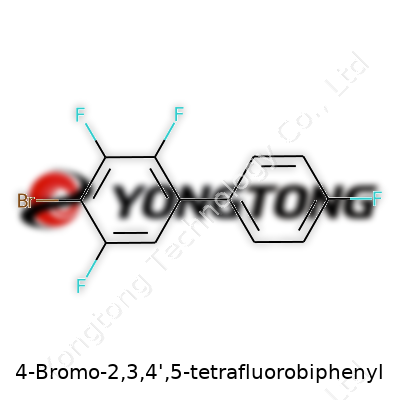
4-Bromo-2,3,4',5-tetrafluorobiphenyl: A Deep Dive into Its Role in Chemistry
Historical Development
The journey of 4-Bromo-2,3,4',5-tetrafluorobiphenyl travels through several decades of fine-tuning organofluorine compound synthesis and aryl halide chemistry. Looking back, the first waves of interest in polyfluorinated biphenyls rose as industries and research institutions examined the unique properties that heavy halogenation brings to aromatic rings. Chemists in the mid-to-late 20th century experimented with halogenation to push the boundaries of molecular stability and reactivity. The push to create fluorinated biphenyls with tailored bromo substitution happened somewhere between the development of cross-coupling reactions, like Suzuki or Stille couplings, and growing interest in designing novel building blocks for electronic materials. Current research into functionalized biphenyls owes a nod to those early trials with selective halogenation.
Product Overview
4-Bromo-2,3,4',5-tetrafluorobiphenyl finds use in advanced chemical syntheses, particularly because its structure offers four fluorine atoms and a bromo group sitting on a biphenyl core. This configuration often draws attention from those working with pharmaceuticals, agrochemicals, and the creation of organic electronic materials, where adding fluorines can change the surface energy, reactivity, or metabolic resistance of molecules. The compound doesn’t crowd shelves in every chemistry lab, but it carves out a spot with researchers chasing tough molecular targets where halogen-and-fluorine interplay matters. In practice, chemists holding a vial of this stuff can either take it further along a synthetic route or study its properties as a unique case in polyhalogenated aromatics.
Physical & Chemical Properties
This compound shows up as a pale solid with respectable thermal stability, thanks to that aromatic backbone and the electronic push-pull between bromo and fluorine atoms. Melting points hover in the region expected for biphenyl derivatives carrying multiple halogens, staying solid at room temperature. Fluorine's strong electron-withdrawing nature makes this biphenyl less reactive toward electrophilic aromatic substitution but better suited to nucleophilic replacements if conditions line up right. The bromine opens doors for palladium-catalyzed cross-coupling and nucleophilic aromatic substitution, which unravels different opportunities than its non-brominated cousins. It dissolves in common organic solvents like dichloromethane, THF, or even DMF for those working at the bench. With a high mass due to its five heavy atoms, all substitutions on the ring influence both physical effects—like volatility or melting—and the electronic structure that drives chemical reactivity.
Technical Specifications & Labeling
When analysts crack open a shipment of 4-Bromo-2,3,4',5-tetrafluorobiphenyl, they expect a documented purity, usually above 97%, with certificates listing water content, residual solvents, and analysis by NMR and mass spectrometry for batch traceability. Labels bring more than a name and batch number—regulatory expectations dictate clear hazard symbols, handling instructions, and reference to safety data sheets. Consistent documentation enables safe handling and helps meet standards in quality control, especially if the material makes its way toward pharmaceutical research. The best suppliers stick to these conventions to avoid labs being caught short if authorities audit their chemical stocks.
Preparation Method
Synthesis of this fluorinated biphenyl often starts with a fluorinated benzene as one substrate and then proceeds through either Suzuki or other palladium-catalyzed cross-coupling methodologies. In research practice, this typically involves preparing a boronic acid or stannane derivative of the biphenyl ring, then coupling with a 4-bromo-2,3,5-trifluorobenzene under carefully optimized conditions—temperature control, inert atmosphere, degassed solvents, and well-chosen ligands all come into play. Some groups might swap in different starting halides for flexibility or greater yield. Researchers often optimize for fewer purification steps since polyfluorinated products can be tough to separate from similar precursors. The presence of multiple fluorines and one bromine pushes chemists to tune electronic effects and avoid over-reduction or unwanted side reactions, and every tweak in procedure can mean the difference between a clean and a messy product.
Chemical Reactions & Modifications
This compound gives chemists options. The bromo group offers a prime handle for further elaboration, such as Suzuki-Miyaura couplings to attach new aromatic groups, or Sonogashira cross-couplings to introduce alkynes. The tetrafluorinated motif also reacts with nucleophiles under the right conditions, making nucleophilic aromatic substitution at activated fluorines possible. With so much electron-withdrawing influence from fluorines, the aromatic system’s reactivity drops in typical electrophilic attack, but what it loses there, it gains in stability and resistance to metabolic degradation. Modifications to the core depend on the intended application—sometimes chemists exchange the bromine for a functional group; sometimes, it serves as a leaving group in metal-catalyzed cycles. The structure’s rigidity and planarity keep crystalline and solid-state applications in play, while multiple halogens adjust lipophilicity or electronic properties in complex syntheses.
Synonyms & Product Names
While catalogues and chemical databases stick with the precise IUPAC name, researchers might call it 4-Bromo-2,3,4',5-tetrafluorobiphenyl or abbreviate for efficiency during meetings or in lab books. Major chemical suppliers, such as Sigma-Aldrich or TCI, reference the full name along with alternative codes, and some publications refer to it simply by a compound identifier or modified biphenyl. CAS numbers solidify identity and avoid confusion in multidisciplinary projects or regulatory review. Such consistency helps track the substance throughout reports, syntheses, and analytical studies, ensuring the right material gets into the right hands.
Safety & Operational Standards
Handling this compound means taking chemical safety seriously. Like most organohalogens, there’s an expectation for gloves, eye protection, and fume hood use because dust or solvent residue can cause irritation or worse if mishandled. Safety data reveals few acute hazards under typical lab use, but the long-term effects of persistent exposure to polyfluoroaromatic compounds remain under investigation. Disposal should follow environmental guidelines for halogenated organics. In my own lab, we bag all fluorinated waste for specialist collection, well aware that improper disposal can mean fines or environmental consequences. Inspections often highlight the need for airtight storage and clear labeling, so confusion between similar-looking white solids doesn’t slow down research or create safety lapses. Documentation practices, logbooks, and training reinforce a culture of caution, especially for new staff learning to respect both common and specialty reagents.
Application Area
4-Bromo-2,3,4',5-tetrafluorobiphenyl claims its real-world value as a synthetic intermediate for pharmaceuticals, agrochemicals, and advanced materials. Researchers studying persistent organic pollutants, flame retardants, or specialty polymers find polyfluorinated biphenyls especially interesting due to their enhanced chemical and thermal stability. In organic electronics, the biphenyl core gets used as a rigid linker or surface modifier, changing charge transport or tuning interfaces in organic semiconductors. Medicinal chemists sometimes introduce multiple fluorines to boost a lead compound’s metabolic persistence or bioavailability. In practical R&D, this molecule serves as a springboard: every new reaction attached to its framework broadens the family of molecules available for drug discovery or material science.
Research & Development
Laboratories across the globe push the limits of what’s possible with polyfluorinated biphenyls, investigating new synthetic routes, environmentally friendly catalysts, and downstream transformation. In our group, we spent months benchmarking different cross-coupling ligands and solvents to improve yields and simplify purification. Continuous flow methods catch my eye—some colleagues swap out batch protocols for microreactors to cut down on waste and maximize throughput. Publications keep growing in journals like Chemical Science and the Journal of Organic Chemistry, where researchers present new derivatives, mechanistic insights, and applications for these molecules beyond standard roles. Funding often flows toward those offering green chemistry solutions—routes with less hazardous waste or recyclable metals pick up momentum as sustainability puts pressure on traditional organic synthesis.
Toxicity Research
Polyfluorinated aromatics, including this biphenyl, raise flags for environmental and human toxicity. No one should ignore growing evidence that persistent halogenated compounds don’t break down easily in the wild. Studies on similar molecules link them to bioaccumulation and potential endocrine disruption, although direct evidence for this specific biphenyl sits behind the known risks for polyhalogenated biphenyls in general. Standard toxicology protocols require in vitro and in vivo testing, with regulatory agencies often erring on the side of caution for fluorinated organics. In our safety briefings, the rule is “don’t release, don’t inhale, don’t taste”—treat every new material like a potential long-term hazard unless conclusive proof says otherwise.
Future Prospects
Looking ahead, the future for 4-Bromo-2,3,4',5-tetrafluorobiphenyl ties into broader shifts in chemical manufacturing and molecular design. Demand keeps rising for smarter, more sustainable ways to install fluorines, as well as better recycling routes for spent or wasted material containing heavy halogens. As organic electronics and next-generation pharmaceuticals seek tougher, more resilient backbones, materials like this one will see continued demand. There’s energy around machine learning as a guide for predicting reactivity or toxicity, simplifying the hunt for safer alternatives or optimization protocols. Green chemistry and circular production methods stand as the next frontier—those who adapt strong environmental stewardship will pave the way for the next wave of research. For all its complexity and risk, this biphenyl reflects both the promise and challenge chemists face when shaping the next chapter in molecular science.
Chemistry, Simplicity, and Real-World Relevance
I remember spending long hours poring over chemical structures, searching for that perfect match between atoms and intent. 4-Bromo-2,3,4',5-tetrafluorobiphenyl isn’t a name that rolls off the tongue, but behind it sits a web of chemical connections. Think about that for a second—a name tells a precise story, and decoding it helps scientists unlock molecular power. Knowing a compound’s molecular formula, for instance, allows researchers to assess its potential risks, biology, and uses long before it’s ever mixed in a laboratory glass.
Dissecting the Structure
Here’s how the molecule breaks down: start with biphenyl, two benzene rings bonded together. Next, layer on specific atoms. Attach a bromine atom to the 4-position of the first ring. Add fluorines to the 2- and 3-positions on that same ring, then add fluorines to the 4' and 5-positions of the second ring. This arrangement doesn’t just change the way the molecule looks—it changes everything, from reactivity to environmental impact.
After counting those atoms, the molecular formula emerges as C12H5BrF4. Twelve carbons, five hydrogens, one bromine, and four fluorines. No excess, no missing pieces. This formula packs a punch in the scientific world. It is more than just a tally—it's a shortcut to understanding everything from toxicity to what happens if this molecule gets into soil or the bloodstream.
Why Getting the Formula Right Matters
Anyone who has experienced a setback in a research project knows how a single mistaken atom throws off weeks of work. Researchers tracking molecules like this often face challenges: safety, purity, environmental burdens. Heavy halogens like bromine and fluorine don’t disappear easily; they persist in water and air. Certain biphenyls, especially the halogenated kinds, live long lives in ecosystems, accumulating in fish or soil. If regulators or scientists miss this detail, people downstream face trouble. It makes sense, then, to emphasize accuracy in everyday research and reporting.
For industrial applications—pharmaceuticals, organic electronics, or specialty chemicals—knowing how many fluorine atoms changes everything. Four fluorines and a bromine mean big changes in electronegativity, solubility, and biological activity compared to plain biphenyl. Forgetting one atom can either ruin a drug’s effect or accidentally create a new pollutant. That’s a big reason formulas get double-checked, every time.
Supporting Responsible Science
It always surprises me how small errors cause lasting problems. Open databases sometimes list incorrect structures or formulas, leading to copy-paste errors that ripple out across journals and patents. To avoid this, researchers lean on verified sources: CAS number records, peer-reviewed journals, and trusted chemical supplier databases. Transparency and strong documentation practices don’t just boost science—they protect public health and the environment. It pays to cross-check, share corrections, and help others spot errors early on.
Moving Forward: Fact-Checking in Every Lab
Decoding a formula like C12H5BrF4 teaches more than chemistry. It shows that precision shapes outcomes in research, medicine, and environmental protection. Pushing for strict, up-to-date documentation, peer review, and honest error reporting can help catch mistakes before they leave the lab. Science, like any craft, always circles back to careful attention to the smallest details.
The Workhorse of Modern Chemistry Labs
You won’t find 4-Bromo-2,3,4',5-tetrafluorobiphenyl in your average household, but inside research labs and chemical plants, this molecule’s reputation is well-earned. A close look at its structure — bolstered by both bromine and multiple fluorine atoms — signals a chemical built for more than shelf decoration. Every researcher knows how picky some molecules are, but this one offers the kind of versatility that serious chemists look for. It stands out for doing some heavy lifting in areas that deserve attention.
Pharmaceutical Research And Fine Chemicals
Drug discovery always runs into roadblocks, especially during late-stage functionalization. With 4-Bromo-2,3,4',5-tetrafluorobiphenyl, scientists deal with a scaffold that responds well in cross-coupling reactions. Its bromine sits ready for Suzuki or Stille coupling, letting researchers swap in other groups. That saves precious time fine-tuning molecules for efficacy or safety. Pharmaceuticals crave this sort of adaptability — small tweaks, big results. These derivatives often bring better metabolic stability or increased activity to test tubes before they ever reach animal studies.
Organic Electronics And Advanced Materials
Chemists and engineers working in advanced materials lean on complex biphenyl compounds to build custom organic semiconductors. Fluorinated rings, especially in biphenyls, offer strong electron-withdrawing power, which makes them ideal in organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs). The bromo-substituted biphenyl provides a starting point for building larger, even more specialized molecules. My time working with thin-film transistors showed how crucial precise molecular structures are. You replace one atom, changing the whole device’s performance. Companies chasing brighter, more efficient screens often reach for compounds like this because the chemistry leads to more stable, efficient products.
Synthesis of Agrochemicals
Less talked about, but no less important: crop protection. Agrochemical researchers tend to start with tough, stable biphenyl frameworks since they hold up against sunlight, rain, and soil microbes. That resilience becomes even stronger when packed with fluorines. Small modifications to the core let designers shape new herbicides or fungicides, making farmland more productive and crops more reliable. I’ve worked alongside teams screening libraries of such molecules to weed out those with unwanted environmental side effects — and it’s clear that starting from a reliable biphenyl makes the process more efficient.
Sustainable Chemistry and Future Prospects
There’s a growing push for cleaner reactions using less wasteful reagents, especially with fluorinated aromatics. 4-Bromo-2,3,4',5-tetrafluorobiphenyl gives process chemists an edge because it participates quickly in well-known cross-coupling reactions. Using these methods, labs can generate target compounds with better atom efficiency and fewer byproducts. This saves both resources and disposal costs. Recent literature suggests new catalysts working well with highly fluorinated aromatics, which might make downstream chemistry even faster and safer. Chemical supply companies improve their offerings each year to keep up.
Looking Downstream
Whether the final product lands in a new drug candidate, a display screen, or a pesticide, the path starts with careful chemistry. Reliable molecules like this biphenyl variant help push projects forward because they respond well under many conditions. For chemists, this land of opportunity comes with responsibility: choosing starting materials that deliver performance, safety, and longevity, all at the same time. With pressure mounting to deliver new solutions in technology and agriculture, no one working at the bench can afford to ignore these building blocks.
A Personal Take on Chemical Storage
I remember the first time I opened a box of specialty chemicals in a university lab. The thrill of it stood shoulder to shoulder with a chunk of anxiety—someone’s carelessness on a different day could’ve made that package useless, dangerous, or simply gone. For people who work with 4-Bromo-2,3,4',5-tetrafluorobiphenyl, a compound valued in advanced chemistry research, these concerns pop up every day. Mishandling won’t just torch your budget, it could pose real health risks.
Proper Storage: Not Guesswork
Safety manuals and big pharma labs agree on the basics, and it all starts with temperature. This compound doesn’t tolerate heat or humidity. Storing it at room temperature, away from direct sunlight, makes a real difference in stability. On paper, this means somewhere around 20–25°C. Let that inch up or down much with the seasons, and the chemical could degrade or even react with air and moisture from the room.
Light protection often gets skipped, especially in crowded, overstressed labs. Sensitive halogenated biphenyls break down over time if exposed to bright light, especially UV. It’s worth the extra dollar for an amber-glass bottle, tucked deep in a cabinet behind a closed door. Remember, breakdown products may escape detection at first, but a single botched experiment shreds weeks of work.
Humidity isn’t just uncomfortable for people in the lab; it shortens the lifespan of many compounds. Keeping bottles tightly closed with a secure seal blocks moisture-laden air from creeping in. Throwing in a small silica gel pack works wonders, especially in a facility without environmental controls. Labs in humid regions often set up dedicated dry cabinets—this approach pays for itself by safeguarding not just one, but hundreds of chemicals.
Labeling, Segregation, and Real Experience
Scrawled labels and ambiguous codes compound confusion. Clear, visible labeling—compound name, date received, and your initials—prevents dangerous mix-ups. I can personally recall my own panic on finding two nearly identical vials in the same drawer from a previous researcher. Take the time to separate halogenated aromatics from acids, bases, and oxidants. If they mix, they don’t just risk spoiling—unexpected reactions might release toxic fumes or start a fire.
Accidents in the workspace almost always result from humans smartly cutting corners. It costs nothing to preach caution, but oversights still crop up. Reporting spills immediately and double-checking seals before leaving for the day keeps everyone safe. Medical journals flag long-term health effects from repeated exposure to biphenyl derivatives, even in trace amounts; so, safety measures aren’t theoretical, they protect your lungs and your future.
Better Storage, Fewer Headaches
Technological fixes are out there, but they don’t replace discipline. Automated monitoring systems work in large organizations, sending alerts at the first temperature blip or open-door event. In resource-limited settings, a careful checklist and routine inspections make the biggest impact. Storage wisdom—simple as a cool, dark, dry place and a well-sealed cap—avoids wasted materials and health scares. Chemical safety, after all, springs from daily diligence, not technological wizardry.
I carry scars from learning these lessons in real time. With each new compound, a fresh opportunity arises to keep standards tight and avoid regret. Careful chemical storage isn’t bureaucratic flourishes—it’s the shield between a good day in the lab and a disaster.
Getting Honest about Chemical Safety
A lot of people only pay attention to chemical names if they show up in the news or trigger regulatory alarms. Yet, the world of specialty chemicals is brimming with compounds like 4-Bromo-2,3,4',5-tetrafluorobiphenyl. Its name alone might send a shiver down the spine of anyone without a chemistry degree. Over the years writing about chemicals and their effects, I’ve learned that it’s not the length of the name that counts, but how it affects people and the environment.
What’s Known about Toxicity and Hazards
Information on this specific compound doesn’t jump out of research libraries or chemical safety sheets, which is a worry. What we do know: the structure fits within the biphenyl family, laced with halogens (bromine and fluorine). Similar chemicals have a reputation—polychlorinated biphenyls (PCBs) devastated both ecosystems and public health. Years after PCBs, we’re still digging toxic residues from riverbeds and looking at studies linking them to cancer, immune suppression, and endocrine disruption.
Nothing suggests that 4-Bromo-2,3,4',5-tetrafluorobiphenyl should get a clean bill of health. Those fluorine and bromine atoms raise red flags. They tend to stick around—persistent, bioaccumulative properties become more likely with each halogen loaded onto the molecule. Chemists understand that fluorinated aromatics often escape breakdown processes, moving up food chains and collecting in organisms. Unpleasant surprises don’t show up right away. Scientists used to think perfluoroalkyl substances (PFAS) were safe. Now lawsuits and contamination reports pile up, not to mention damaged trust in chemical oversight.
Exposure Risks: In the Lab and Beyond
Most people walking down the street won’t cross paths with this compound. Still, researchers, manufacturers, and waste handlers could face exposure. Handling unknowns without up-to-date toxicology can be a costly gamble. Skin contact or breathing dust can lead to problems, even for seemingly harmless chemicals, and biphenyl derivatives sometimes irritate, sensitize, or worse. Inhaling powders often creates bigger risks than touching solids—tiny particles shoot deep into lungs and sometimes cross cell membranes.
In my experience, even unregulated specialty compounds deserve gloves, goggles, and a fume hood. Newer isn’t always safer. Sometimes, the industry moves quick, racing for new materials while regulatory science limps behind. Engineers and project leads need to chase down supplier safety data sheets and demand clear answers before letting anyone open a new drum.
Healthy Skepticism and Real Solutions
People downstream from chemical plants hope buyers and users ask tough questions. Environmental persistence means today’s shortcut can show up in drinking water years later. Manufacturers should fund independent toxicity screens, not just regulatory minimums. If something looks or acts like persistent halogenated biphenyls, treating it with extra respect often proves wise. Some industry groups now share data with environmental researchers to fill gaps left by old regulations.
Preventing harm calls for shared vigilance—engineers, buyers, regulators, and nearby communities alike. It costs less to prevent a mess than to clean one up years later. Treating 4-Bromo-2,3,4',5-tetrafluorobiphenyl with the same caution reserved for its infamous cousins isn’t alarmist—it reflects lessons hard-learned over decades of chemical mishaps.
Understanding Purity Standards
Quality makes all the difference in the chemical world, especially when dealing with advanced organic synthesis like 4-Bromo-2,3,4',5-tetrafluorobiphenyl. Labs and production facilities gravitate to purity levels that make experiments repeatable and outcomes reliable. This compound regularly appears at 97% purity or higher. A clear lab report, usually from HPLC analysis, gives customers confidence in what they’re buying, and a high-purity batch means fewer headaches chasing down side-products or mysterious spots on TLC plates.
Getting sloppy with purity carries real risks—whether you worry about false starts in research or trouble downstream if the impurities stick around. Some chemists even hunt for ultra-high purity, but most applications, such as pharmaceutical R&D, electronic material research, or advanced coatings, work with the 97% to 99% range. Higher cost accompanies higher purity, but cutting corners here often means paying more later.
Available Packaging Sizes
Visit any reputable supplier, and you’ll notice their focus on meeting different needs with flexible packaging. The smallest packaging usually starts at 100 milligrams. This gives researchers room to run a couple of reactions or screening tests without breaking their project budget. For those scaling up or running extensive analyses, 500 milligram and 1 gram bottles exist for easy access.
Industry or institutional buyers who commit to routine synthesis sometimes need more than a few grams. Leading suppliers will fill custom orders, and it isn’t strange to see 5 grams, 10 grams, or even 25 grams offered upon request. Extensive paperwork, special labeling, and careful transport protocols often shadow bulk shipments, especially with specialty halogenated aromatics like this one.
Significance of Proper Packaging
Anyone who has suffered through a leaky cap or poorly labeled vial can relate to the headaches poor packaging causes. Glass vials with secure PTFE-lined caps guard against contamination from moisture and air. Every milligram counts, and careless handling turns pristine white powder into a frustrating mess or even a safety concern.
Regulatory bodies, including OSHA and the European Chemicals Agency, push for robust packaging and clear safety data with compounds like this. Strong labeling, batch numbers, storage conditions, and expiry dates make traceability possible if ever called into question, either during routine audits or after unexpected lab incidents.
Improving Access and Transparency
Better transparency from suppliers would go a long way in chemical sourcing. Detailed certificates of analysis with every shipment, openly displayed impurity profiles, and lot histories support good science and safe handling. More suppliers are starting to publish their batch data online. Users benefit from upfront details—purity, water content, even residual solvents—without jumping through hoops to get them.
Open communication between buyers and vendors opens the door to tailored solutions. Whether it’s a unique aliquot size for a multi-year study or expedited shipping in temperature-controlled containers, a little flexibility builds long-term trust. One size never fits all in science; product adaptability and rock-solid reliability create an environment where innovation, not procurement, drives the project.