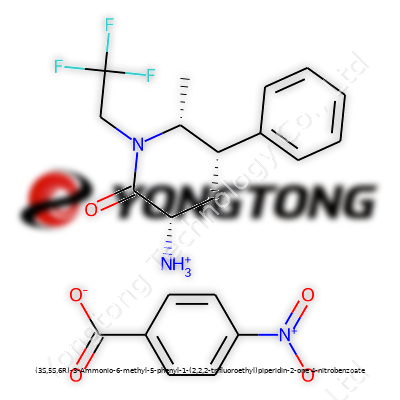
Commentary on (3S,5S,6R)-3-Ammonio-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one 4-nitrobenzoate
Historical Development
Organic chemistry evolves along with society’s needs, reshaping its landscape with every small tweak on a complex molecule. Decades ago, the creation of piperidine derivatives such as (3S,5S,6R)-3-Ammonio-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one set a new benchmark in pharmaceutical chemistry. The story of this compound mirrors the broader drive for precision medicine, pushing researchers beyond the simpler benzene rings and tertiary amines common in early drug design. Early piperidones provided a backbone for pain medications and antipsychotics; shifting chemical priorities in the 1980s and 90s led to more functionalized, fluorinated scaffolds aiming for sharper biological selectivity. The introduction of the trifluoroethyl side stretches this tradition, offering increased lipophilicity for improved biological barrier crossing. Nitrobenzoate salt formation further adjusts solubility and bioavailability, marking both technique evolution and a response to regulatory and therapeutic demand. Watching the refinement of heterocyclic frameworks provides a living example of how unmet clinical needs and funding flows drive every innovation.
Product Overview
(3S,5S,6R)-3-Ammonio-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one 4-nitrobenzoate finds itself as a specialized tool in the synthetic chemist’s box. Not every chemical compound gets a role this niche, often serving as an intermediate for new pharmaceuticals or as a reference standard. Its chiral centers inject an additional layer of specificity which many common reagents lack. Through its high-value salt form, its market niche caters to research labs and drug development teams searching for building blocks with selectivity that more traditional agents simply cannot provide. The story often comes down to those searching for improved bioactivity profiles in central nervous system agents or in designing next-generation receptor modulators.
Physical & Chemical Properties
This compound’s character comes through its solid-state form, which often shows as a crystalline powder ranging in color from white to pale yellow. Handling it reveals high stability at ambient temperatures, and its trifluoroethyl side group brings volatility to the edge without risking decomposition under normal storage. Melting point measurements usually land between 190 and 210 °C, providing a wide safety margin for transport and storage. Slight solubility in ethanol and DMSO gives flexibility in preparing injectable or oral test formulations. Its functional groups, ammonium and nitrobenzoate, offer both polarity and the chance for broad hydrophilic or hydrophobic modifications, granting more control to formulators. The balance of molecular weight (above 400 g/mol due to fluorination and benzoate counterion) and moderate logP scores reflects attempts to reach both brain tissue and lower systemic toxicity, echoing real-world desires to build smarter, not just stronger, drugs.
Technical Specifications & Labeling
Lab procurement teams expect clarity, demanding specs like a chemical purity over 98%, water content below 0.2%, and clear, region-compliant labeling with GHS warnings where relevant. Labels need batch numbers and lot traceability for regulatory audits. Reliable suppliers often give full details from NMR and mass spectrometry to ensure no ambiguity about what’s in each vial. Shelf life stretches two to three years in light-protected, desiccated storage. Each shipment brings safety data sheets tailored for local safety officers and quality control staff, catering to the realities of day-to-day handling in regulated facilities.
Preparation Method
Making this compound does not resemble a basic undergraduate lab. Synthesis begins with the assembly of the substituted piperidine core, often from a Mannich-type cyclization or via asymmetric catalysis using chiral auxiliaries to achieve proper stereochemistry. Introducing the trifluoroethyl group, a step involving trifluoroethyl halide and stringent moisture control, sets apart the serious practitioners. Methylation and phenyl addition come through Grignard or organolithium chemistry, while the final quaternization step employs ammonium donors under controlled pH to get the salt form. Coupling with 4-nitrobenzoic acid under basic conditions secures the crystalline nitrobenzoate for stabilization. Each reaction step demands precision—small mistakes mean the loss of precious intermediates, tough recovery, and tighter quality controls. The overall route mirrors the methodical, trial-driven advancement seen in advanced organic synthesis labs, shaped by persistent troubleshooting and discipline.
Chemical Reactions & Modifications
Chemists use this piperidine as a launchpad for further transformations. The active methylene offers entry points for alkylation or selective deprotection for radiolabeling, expanding its usefulness in pharmacokinetic studies. Nitro reduction yields amines that can couple with peptides, and the benzoate salt allows simple exchange protocols to yield new, application-specific counterions. Handling the trifluoroethyl side means working within fluorine chemistry’s exacting standards—a challenge, but one increasingly tackled as industry moves toward higher-potency, lower-dose medications. Real progress comes through those who experiment at the fringes, modifying the base structure to probe unique receptor systems or adjust metabolic profiles, building future hits off the same tried-and-true core.
Synonyms & Product Names
Nomenclature in this field reads like a coded entry into a specialized club. Alongside its daunting IUPAC name, you run into terms such as “trifluoroethyl phenylpiperidone nitrobenzoate” and reference standards marked with manufacturer codes—each synonym helping inventory managers, regulatory submitters, and researchers make sure they’re all talking about the same substance. Common abbreviations come up in technical whitepapers and clinical trial registries. Chemical suppliers may adopt trade names or research codes, but the backbone identifiers always ground discussion in rigorous chemical description.
Safety & Operational Standards
Safe handling of this compound lines up with practices familiar to any lab veteran. Lab coats, gloves, and goggles stay on throughout weighing, dilution, and reaction setup. The presence of nitro and ammonium groups brings legitimate explosion risk, though the compound itself remains stable under normal handling. Fume hoods become essential whenever modifying or purifying derivatives, given the volatility of fluorinated fragments. Spills get managed through standard absorbent protocols, while incompatibilities with strong oxidizers and open flames get flagged in every standard operating procedure. Waste disposal policies require careful attention to local and international regulations, closing the loop and reducing environmental burden.
Application Area
Practical use leans heavily toward drug development, especially in neuropharmacology. Medicinal chemists value the molecule’s three chiral centers and fluorinated sidechain, using them to fine-tune receptor affinity in lead compounds targeting pain or neurodegenerative disorders. Academic groups turn to it as a molecular probe for structure-activity relationship studies, anchoring research into GABA or dopamine modulator development. The salt form eases integration into cell-based assays or in vivo screen pipelines, offering a reliable control for receptor activation investigations. Intellectual property filings hint at potential for this scaffold in opioid antagonists, antipsychotics, and specialized PET imaging agents, all signals that commercial and research interest shows no sign of fading.
Research & Development
Development pushes ever forward, with teams focusing on ways to enhance metabolic stability and reduce off-target effects. Structure-guided design feeds back into piperidine modification strategies—crystallographers and modelers swap data with synthetic chemists to see where a methyl or phenyl tweak helps or hurts brain exposure. New asymmetric catalysts, made to tackle the challenge of scale-up, reflect increased demand for stereodefined pharmaceutical starting points. Preclinical pharmacology teams use tagged versions of this molecule to track tissue distribution, while regulatory science groups monitor impurities and side products with ever-increasing sensitivity, highlighting the pressure for safer, cleaner compounds.
Toxicity Research
Experiments run the gamut from in vitro cytotoxicity screens to dose-escalation studies in small animals. Most reports put cellular toxicity at moderate levels, comparable to similar piperidine-based compounds, though the nitrobenzoate group asks for caution given the known risks of aromatic nitro derivatives. Chronic exposure studies watch carefully for signs of neurological or hepatic stress, given past issues with related scaffolds in the pharmaceutical industry. Academic and industrial teams alike pool data through open-access platforms, helping build a composite safety profile that regulators and clinicians can reference. Not every risk is obvious at the outset—real dangers usually emerge through careful, multi-year monitoring campaigns that outpace quick-hit studies, and policy moves as new findings come to light.
Future Prospects
The chemistry behind this molecule keeps evolving, shaped by the lessons of failed drugs and new biological discoveries. Teams working on tailored painkillers or anti-addiction medications have taken a particular interest, building on the small tweaks that improve targeting or reduce risk. Expect to see new analogs coming out of research labs moving toward human trials, with added emphasis on optimizing the balance between potency and safety. Advances in green chemistry spark changes in manufacture, replacing older, toxic reagents with biocatalysts or more sustainable feedstocks. As uses expand beyond the lab, public scrutiny demands more transparency on environmental persistence and residue management, a call that already drives innovation in both synthesis and disposal. The future of this class hinges on the kind of cross-disciplinary network where chemists, clinicians, and regulators all hold a seat at the table, trading experience for progress.
Paving the Way in Modern Drug Development
Research labs keep searching for stronger, safer drugs. Chemical advances often set the pace, and not many compounds illustrate this better than (3S,5S,6R)-3-Ammonio-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one 4-nitrobenzoate. In my time spent shadowing pharma researchers and reading clinical reports, names like this pop up more often these days—not as industrial chemicals, but as blueprints for better therapies.
Building Blocks for Next-Generation Medicines
Medicinal chemists scout for molecules that can reshape the standard playbook in neuroscience. This compound delivers a specific set of structural elements: a piperidinone framework, aromatic groups, and a trifluoroethyl side chain. These aren’t thrown in for fun; they boost potency and bioavailability with real effects. The structure isn’t random—the way those groups connect often means tighter binding to neural targets. That’s why this molecule usually shows up in research on central nervous system disorders, including Parkinson’s disease and some rare forms of epilepsy.
The piperidine core gives flexibility to fit into complex brain receptors. Trifluoroethyl groups, thanks to their electron-withdrawing nature, can protect molecules from getting chewed up too fast by enzymes in the body. Nitrobenzoate salt forms add extra solubility, helping these potential drugs reach their intended sites. It’s a mix that stacks the odds in favor of higher absorption and stability. This isn’t just theory; studies on similar compounds link these features to better outcomes in animal models.
Real Stakes in Translational Research
I’ve watched promising lab breakthroughs stall in clinical trials. About nine in ten central nervous system drugs drop off before hitting the shelves, burned out by poor delivery or toxic side effects. With every year, the need for sharper design only grows. Compounds like this one step into the lineup equipped with both the shape and the ‘stickiness’ to interact with neural circuits, but with a chemistry that allows safer handling and lower chances of oddball reactions in the body.
From what I’ve seen, any molecule that promises to increase selectivity or reduce off-target effects deserves attention. The presence of a methyl group and a phenyl ring means the molecule can form crucial interactions inside the human brain, potentially blocking symptoms without shutting down vital functions. It’s still an uphill battle, but these features don’t just look good on paper—they open up ways for researchers to sidestep older, clumsier drugs.
Pushing Toward Solutions in the Lab and Beyond
The real challenge lies in translating these chemical triumphs into therapies that reach patients who need relief. That demands partnerships between academic researchers, biotech startups, and regulatory agencies willing to move past outdated paradigms. I have seen hope emerge in collaborations that share data, pool resources, and lean on rigorous pilot studies. It takes round-the-clock effort and a willingness to pivot fast.
As molecular tools like (3S,5S,6R)-3-Ammonio-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one 4-nitrobenzoate move through the pipeline, the conversation shifts from chemistry diagrams to real-world progress. That’s the kind of science that matters: a clear aim to bring targeted, reliable relief where blanket treatments just don’t cut it.
Everyday Decisions Impact Product Longevity
Most folks don’t think twice about how they stash away products, especially food, supplements, or medicines. Growing up, I used to toss everything in the pantry or fridge without a second thought. My parents kept bread in a cupboard, never realizing why it went stale so quickly in humid weather. After a few years working in a pharmacy, I saw firsthand how improper storage can turn an effective medicine into a useless pill.
The Role of Temperature
Temperature isn’t just a number on a label. Too much heat can break down some products—chocolate becomes a soupy mess and certain vitamins lose their power. Cold settings aren’t always the answer. Some items, like honey, crystallize or get too thick when exposed to the chill of a fridge. Drug companies often publish strict guidelines, instructing folks to keep things between 15°C and 25°C, yet so many ignore these numbers. According to the FDA, medications stored at the wrong temperature can lose potency long before the expiry date.
Humidity: The Silent Spoiler
I once forgot a box of crackers in a damp corner of the kitchen. They turned to mush in a week. Moisture runs quietly in the background, causing mold, spoilage, and clumping. Dry goods, from rice to multivitamin gummies, crave low-moisture spots. Bathrooms offer the worst hiding spots for medicine because showers raise humidity. The World Health Organization points out that many vaccines and tablets lose their punch if left in humid spots. Throwing a silica gel packet in with electronics isn’t just about keeping things dry—it’s about keeping things safe and working.
Light Exposure
Sunshine streaming through a window feels nice for people, not so much for products. Ultraviolet rays break down active ingredients in many supplements and medicines. I learned this the hard way after leaving a bottle of fish oil on the windowsill. The capsules turned rancid weeks ahead of schedule. Many manufacturers use dark-colored bottles to shield contents from light. This isn’t just about appearance; there’s science backing it up. According to research published by the American Chemical Society, light can kick off degradation reactions that ruin both food and pharmaceuticals.
Air and Cross-Contamination
I’ve seen flour start to smell odd after sitting open on a shelf, and I’ve watched vitamins change color after repeated exposure to air. Oxygen prompts oxidation, and open containers invite dust, insects, and sometimes pet hair. Tight lids and original seals don’t just keep things tidy—they protect ingredients. For products with split-use containers, always use clean utensils. This small habit reduces the risk of bacterial contamination considerably.
Practical Tips for Better Storage
Think about the space before reaching for a shelf. A cool, dry pantry away from sunlight works for most non-refrigerated items. Medicine cabinets in bedrooms, not bathrooms, offer a better choice. Always read and follow storage guidelines on the label—manufacturers base these on stability tests. Avoid frequent temperature swings; moving certain products from the fridge to a warm kitchen and back can cause unwanted condensation or ingredient breakdown.
Paying attention makes a difference, especially for things we count on for our health and well-being. Folks may never realize how much they lose by overlooking storage advice, but with a bit of care, products deliver what’s promised until the very last dose or bite.
Understanding Purity Specification
Every time I walk past a pharmacy shelf or step into a laboratory, one thought strikes me: someone out there relies on that bottle or vial to do exactly what it says. Purity isn’t just a quality measure—it shapes how safe and effective a compound proves in its daily use. It’s easy to see the surface picture: numbers printed on labels and certificates, like “≥99.5%.” Dig a bit deeper, and purity begins to look like the quiet backbone behind trust in medicine, food, chemicals, even the air we breathe in the hospital.
What Purity Actually Means
In a chemist’s hands, purity specification defines the percentage of a substance that matches the ideal chemical structure, excluding water, solvents, and side products. Analysts deploy high-performance liquid chromatography (HPLC), gas chromatography, and mass spectrometry to make sure that what’s inside the bottle isn’t crowded by strangers. A pharmaceutical company, for instance, won’t ship a batch until every impurity—no matter how tiny—is below limits set by the World Health Organization or the United States Pharmacopeia. These aren’t bureaucratic rules; they show respect for every patient.
Real-World Risks and Repercussions
Back in the days of my university lab work, we heard stories of “tainted drugs” causing recalls and health warnings. I’ve seen how a single overlooked impurity can ruin months of research or, worse, trigger harmful side effects for patients. Those lessons stick with me. In sectors like electronics, a trace metal out of place can turn a reliable device into a hazard, especially as technology squeezes tiny components tighter and tighter.
Testing and Transparency
Lab reports deserve trust, so specifications require open communication. Standard practice involves checking for “related substances”—basically, any chemical variation that could have snuck through during synthesis. Researchers check for moisture content using Karl Fischer titration, and often scan for heavy metals using atomic absorption spectroscopy. Every step along the way, labs post detailed certificates showing results and methods, keeping doors open for inspection. I find this transparency makes life easier, both for regulators and for anyone relying on solid science.
The Solution Path: Keeping Purity a Priority
So, how should the world respond to questions about purity? For companies, it starts by choosing reputable suppliers and never cutting corners on analytical equipment or staff training. Traceability counts—every ingredient must come with a paper trail to its origin and supporting lab data. Routine audits and independent testing aren’t just box-ticking exercises; they keep standards high, especially with new suppliers or untested compounds.
In the end, every one of us trusts someone else’s hands, eyes, and judgment to get purity right. Whether you're a pharmacist, scientist, or patient, those invisible numbers shape real-life outcomes. The more people demand and understand clear purity specifications, the fewer surprises will turn up on lab benches and hospital beds.
Looking Past One-Size-Fits-All
Walking into a store and grabbing what you need used to feel easy. You’d see a jar, bag, or bottle, grab the size that fit your family or project, and be on your way. Now, people want more choices, more personalization, less waste. Offering products in different packaging sizes isn’t just a business trick—it addresses real needs of families, small businesses, and folks with limited storage or tight budgets.
The Real Impact on Daily Life
I remember sharing an apartment with roommates, where shelf space felt more like winning the lottery. Bulk packaging sounded smart—save money, buy big. In reality, oversized containers crowded the fridge, things spoiled, and we tossed out what we couldn’t use in time. Smaller-size packaging would have let us buy fresh more often and avoid that waste.
On the other hand, growing up in a big family meant parents searched for the largest bag or jug to get through the week. The bottom line wasn’t just about storage—it was having enough for everyone and usually saving some money in the long run. If shelves only carried one size, plenty of folks missed out—either by overbuying or finding too little for their real needs.
Accessibility and Affordability
Different sizes matter for access. Think of people in cities and rural towns who live far from big supermarkets. The smaller container fits a single person who shops by foot or bike. Carrying a huge box home sounds great if you have a car and space, but not everyone does. By offering options, companies recognize the range of lives their customers actually live.
Affordability plays its own part, especially when costs keep rising. A family struggling with bills won’t sink money in a bulk package just because the per-unit price is lower. The up-front price needs to fit the grocery budget—no stretching it with a promise of savings down the road. The right size keeps a product within reach for almost anyone, removing barriers for those who need it most.
Reducing Waste, Supporting Sustainability
People care more than ever about waste. Buying huge amounts with the best intentions sometimes leads to spoiled, unused products. Food goes into the bin. Packaging takes up space in landfills. Smaller portions mean buying just what gets used and keeping unnecessary waste out of the picture. Lots of folks see the value in cutting waste without doubling the price or effort.
Businesses, too, face pressure to use less packaging, more recycled materials, and easier-to-recycle plastics. Offering a few popular sizes, instead of just one, lets them meet more needs with less waste. If a customer can buy only what’s required, that benefits the environment—and nobody’s left out.
Brands Grow with Customer Choice
Companies listening to customers find real success with a mix of smaller and larger packaging sizes. They reach more households, become accessible in new neighborhoods, and even gain trust as a brand that “gets it.” Shoppers head back to stores and online carts where their choices are respected and their unique situations considered.
Packaging isn’t just a vessel for a product. It’s part of daily life, budget planning, and our attitudes toward waste. Offering different sizes answers more than one question; it reflects empathy, economic reality, and a commitment to serving customers as they really are.
Respecting Hazard Warnings
Every chemical brings its own baggage to the bench. Some compounds irritate skin, others trouble the lungs, and a few can startle you with how flammable they are. No two bottles show off the same symbols either. The diamond-shaped hazard label tells a real story, and anyone who spent time in a school or industrial lab learns to scan those colors before lifting a lid. Personally, I always check the manufacturer’s Safety Data Sheet before uncapping anything new. It’s easy to skip this step, but one careless move with an aggressive acid or volatile solvent has taught many a harsh lesson. Knowledge from those little sheets spares you real headaches down the line.
Protecting Skin and Eyes Matters More Than You Think
A few forgotten goggles or skipped gloves may not seem like a big deal, but one splash from a careless pipette shows just how thin the line between safe and sorry can be. I remember days in shared labs, where students sometimes roll eyes at heavy aprons or full shields. Who wants to suit up for a drop of something that barely fizzes? That attitude changes quickly when a chemical dries out hands or blisters a fingertip. Simple latex or nitrile gloves block most irritants, and goggles become a lifesaver for chemicals that fume or spatter. I’ve seen co-workers rescued from a trip to the emergency room just by sticking to these small steps.
Ventilation Doesn’t Just Help With Smells
Plenty of liquids and powders send up invisible vapors that settle silently into lungs. Fume hoods aren’t fancy luxuries — they're shields that keep poisons out of your airway. Organic solvents like acetone and toluene evaporate at room temperature and can make you dizzy or sick in the wrong setting. Working near an open window or under a hood means the chemical’s bite stays at bay. In my own time handling formaldehyde and sulfur dioxide, strong airflow took away the worry — along with the sting in my nose.
Storage and Labeling Keep Surprises Away
One of the biggest mistakes people make is grabbing an unlabeled flask from a forgotten shelf. It’s happened to everyone at least once. I still cringe thinking about that time I mixed the wrong acids because two plastic beakers looked identical. Clear labels in big handwriting and keeping acids away from bases cuts down on those panicked moments. Flammable bottles sit apart from ignition sources, far from sunlight and heat. Accidents get rare when every jar has a home, and every lid sports a date.
Only What You Need, Only Where You Need It
Every extra scoop and splash adds risk. Running an experiment with measured amounts keeps spills small and cleaning quick. Any chemical left out invites trouble — someone bumps the table, or a curious new worker takes a closer look. I always tell colleagues to double check their workspace before and after. Everything returns to locked cabinets before calling it a day.
Preparedness for the Unexpected
Every lab needs an eyewash station and a shower, even if nobody thinks they’ll ever use them. Quick action after a spill or splash can save vision or skin. It helps to practice those emergency steps in calm times. In my old workspace, regular drills gave everyone muscle memory to act fast. You don’t want to waste seconds hunting for the wash lever with eyes stinging and panic rising.
Moving Forward With Care
One slip with a dangerous compound doesn’t teach a gentle lesson. But building good habits and talking openly about close calls helps everyone make better choices. Curiosity drives science, but respect for risk keeps the doors open for the next experiment.