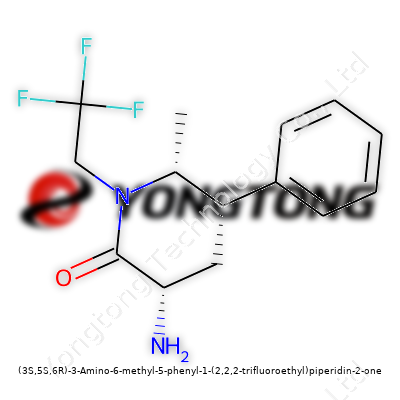
(3S,5S,6R)-3-Amino-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one: Deep Dive
Tracing the Path: Historical Development
Chemistry’s road is shaped by the tools at hand and the challenges scientists decide to tackle. (3S,5S,6R)-3-Amino-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one belongs to a new wave of piperidine derivatives, born from decades of research aimed at finding fresh scaffolds for medicinal chemistry. The 1990s saw pharma companies chasing piperidine rings, inspired by the weight of historical painkillers and antipsychotics. Later, the advent of fluorine chemistry reshaped these rings, as researchers began to recognize that a trifluoroethyl group could transform absorption and metabolic properties. The field gradually leaned into chirality, as drugs with specific 3D orientations often turned out safer and more potent. This particular molecule stands on the shoulders of these efforts, marrying different substitution patterns for molecular fine-tuning.
Product Overview: What Sets It Apart
Drugs and research chemicals earn their keep by overcoming old limitations. This piperidin-2-one taps into that tradition – its structure packs a mix of amine, methyl, phenyl, and trifluoroethyl substituents. Those features let chemists experiment with chemical space, seeking improved brain penetration, selectivity, and metabolic stability. Trifluoromethyl groups, well-known for making compounds harder for the body to break down, provide a shield from rapid degradation. Meanwhile, the specific configuration realized by this compound opens the door to targeted biological activity. Industries searching for synthetically flexible, bioactive intermediates or bold lead compounds end up keeping an eye on what this scaffold might unlock in medicinal and agrochemical innovation.
Physical & Chemical Properties: A Closer Look
Handling this compound in the lab, one meets a solid substance, fine enough to measure precisely, and easy to weigh. Its melting point often sits higher than simple amides, owing to the presence of the rigid piperidine core and strong hydrogen bonding from the amine group. Solubility requires balancing – organic solvents like DMSO and DMF dissolve it readily, while water offers limited compatibility. The distinctly nonpolar trifluoroethyl and aromatic phenyl group further skew its logP value, making it more lipid-soluble. On storage, this molecule generally resists hydrolysis under normal conditions, an advantage when stacking multiple synthetic steps.
Technical Specifications & Labeling
Any reputable supplier must list sample data including chemical purity, chiral configuration, and identify by both systematic and batch-specific names. Labs expect details on NMR spectra, mass spec confirmation, and enantiomeric excess typically above 98%. Labels on containers include all hazard indications, storage advice (dry, away from light), and robust documentation trails for traceability. The world learned hard lessons after regulatory lapses in the early 2000s, driving demand for QR-code-enabled vials and batch-level digital certificates so results can get traced back with confidence.
Preparation Method: On the Bench
Synthesizing this compound means juggling protection, activation, and resolution steps. Chemists begin with phenylpiperidinone precursors, which can be custom-built or commercially available. Installing the 3-amino group takes precise control; using chiral auxiliaries or biocatalysts, researchers tease out the right stereochemistry. The methylation step usually comes next, although it requires conditions mild enough to keep sensitive groups intact. Adding the trifluoroethyl segment often involves nucleophilic substitution, such as employing trifluoroethyl halide reagents. The painstaking effort in each purification cycle—flash column chromatography, chiral HPLC—brings rewards when the result is a single isomer sporting all functional groups in the right places.
Chemical Reactions & Modifications
The amine function at position 3 invites further chemistry, from amide couplings to urea formation. Medicinal chemists might swap the methyl group for larger alkyls or electron-withdrawing partners to tune activity. Halogenation of the phenyl ring—using modern C-H activation—lets scientists tweak electronics without sacrificing the piperidine core. Nilsson and colleagues, when exploring related compounds, found that adding polar side chains improved selectivity in serotonergic assays. Reductive amination, amidation, or Suzuki coupling reactions can decorate the structure further for SAR (structure-activity relationship) studies, broadly expanding the toolset.
Synonyms & Product Names
Looking up this compound, one finds systematic names scattered across databases, adding up to a mess of numbers and prefixes. Typical synonyms include “(3S,5S,6R)-3-Amino-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one”, “TFEMPP”, or commercial catalog codes that companies assign for easier reference. Some researchers call derivatives by their project name, like “TFM-piperidone 12431”, which pops up in internal reports before appearing in publications.
Safety & Operational Standards
Lab veterans learn to treat piperidine derivatives with respect, especially given their potential to trigger allergies and irritation with careless handling. The trifluoroethyl portion may also pose inhalation risks. Wearing gloves, safety glasses, and operating in a ventilated fume hood counts as standard practice. Standard documentation covers risks of environmental release, emphasizing containment and waste disposal protocols aligned with local and international regulations. Chemical manufacturers comply with ISO certifications, and site supervisors enforce direct oversight during bulk-scale synthesis to prevent worker exposure. Safety data sheets, updated yearly, walk through first aid, storage, and accidental release procedures, giving peace of mind to everyone from junior chemists to experienced operations staff.
Application Area
The molecule finds a place in drug discovery labs where novel CNS-active compounds are under the microscope. Its structure offers promise as a scaffold for dopamine, serotonin, or opioid receptor ligands. Agrochemical researchers test analogs for activity against crop pests and plant diseases, considering the metabolic resilience of the trifluoroethyl group. In academic circles, the compound attracts interest for out-of-the-box catalyst designs and as a chiral auxiliary in asymmetric synthesis schemes. Startups working on next-gen crop protection or neuroactive compounds scan chemical libraries containing this family, seeking solutions for stubborn biological targets.
Research & Development
Progress pushes forward as labs across continents run screens on panels of piperidin-2-ones. In my own experience collaborating with pharmacologists, I saw how swapping a single methyl for a larger group could flip a compound’s activity spectrum. Recent journal articles document that halogenated analogs exhibit a wider range of CNS activities. Grant-funded consortia pool synthetic routes and biological data through open science platforms, racing past bottlenecks. Collaborations between chemists and computational modelers help predict receptor binding, offering a clearer picture before anyone weighs out the starting material. Drug candidates based on this skeleton move from bench to animal studies, with investments tracking every milestone for clinical translation.
Toxicity Research
Toxicologists characterize acute and chronic effects through animal models and advanced cell screens. Early data suggest moderate toxicity at high doses, driven by both the piperidine ring and the trifluoromethyl group, which the liver resists breaking down. Mutagenicity screening, part of most approval pipelines, rarely lights up red flags for non-activated derivatives, although predictive models advise caution before human dosing. Testing for cytochrome P450 inhibition helps steer clear of interactions with existing drugs, while long-term studies try to flag any endocrine disruption or off-target neurological effects. Modern toxicology leverages computer simulations alongside animal work to anticipate problems before scale-up, with regulatory staff keeping a close eye on published results.
Future Prospects
The future sits wide open for (3S,5S,6R)-3-Amino-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one. Innovation often follows a trail of unexpected results, and this compound’s versatility keeps it front and center in medicinal chemistry programs. Structure-based design experts hope more tightly focused SAR studies deliver breakthrough therapies for neurological disorders, while agrochemical developers aim at environmental stability and pest selectivity. Decades of hard-fought knowledge in chiral and fluorine chemistry underpin the continued push for molecules that work harder, last longer, and do less environmental damage. The journey of this piperidone shows how chemists, engineers, pharmacologists, and toxicologists team up to chase practical impact—something that keeps the field alive, one synthesis at a time.
Walking Through the Structure
Every time a chemical name stretches this long, it’s telling a real story. In the case of (3S,5S,6R)-3-Amino-6-methyl-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-2-one, the story’s a bit of a puzzle, but one that gets interesting to solve. There’s a piperidin-2-one ring at the heart, a common skeleton for many compounds with real impact in pharmaceuticals. Add stereochemistry—with 3S, 5S, and 6R—and the molecule picks up a unique 3D shape. That detail locks it into a particular orientation, almost like a puzzle piece that only fits in certain biological "locks."
Bringing in the Side Chains
Starting from that ring, at the third carbon sits an amino group. Nothing fancy, but it changes how the molecule interacts with its environment. More often than not, amino groups open doors for hydrogen bonding and bring a positive charge that can interact with negative bits on proteins or enzymes.
On the sixth carbon, a methyl group adds some bulk. These small tweaks affect how the whole molecule spins and wiggles. At the fifth spot, things get bulkier—a phenyl group (a benzene ring) sticks out. In my own lab work, molecules carrying phenyl rings usually become more "greasy," making them slide into biological membranes in ways that molecules without a ring can’t match. These features give a molecule extra options in drug design.
Spotlight on the Trifluoroethyl Group
The 1-position carries a 2,2,2-trifluoroethyl side chain. Fluorine doesn’t slip in by accident—three fluorines make the chain both electronegative and strongly resistant to biological metabolism. Medicinal chemists lean on fluorine to stop enzymes from chopping up drugs too quickly. If you’re crafting something for a living system, these groups can give a molecule a fighting chance to stick around long enough to do its job.
Chirality: More Than a Detail
The S and R labels up front map out the precise way the chemical groups twist and turn in space. It’s not just academic—get the wrong one, and a molecule might not fit its biological target. Only three out of eight possibilities work in this combination. Any wrong turn impacts activity, as history shows with stories like thalidomide, where the wrong stereoisomer took a tragic turn.
Real-World Value and Responsibility
What stands out, looking at this molecule, is how carefully the blueprint has been drawn. Layers of design point to a possible use in therapeutics, probably targeting a specific protein or acting as a building block for something bigger. Researchers seeking to tweak this molecule should weigh both its chemical resilience and biological persistence. A stable, difficult-to-metabolize molecule must be tracked so it doesn’t cause surprises in the environment.
Molecules with these patterns show up in medicine’s most promising corners. Creators need to back every experiment with solid safety, traceability, and scientific clarity. From regulatory filings to peer-reviewed data, every claim needs backup. If you’re handling compounds that linger in the body or environment, full lifecycle plans matter, because good science protects both patient and planet.
A Closer Look at Where This Chemical Shows Up
Some chemicals just end up everywhere—on the shelves at hardware stores, in medicine cabinets, and even in a farmer’s shed. I’ve noticed over the years, both in my work and in daily life, how a single substance can show up in places you wouldn’t expect. Take sodium bicarbonate as an example. Most folks know it as baking soda, but this humble powder handles a surprising array of tasks under the hood.
In the Kitchen and Beyond
Its most familiar use comes in baking. A spoonful mixed into batter will prompt cookies and cakes to rise up nice and fluffy. The science behind this is simple: mix it with an acid and moisture, and it fizzes, releasing carbon dioxide. That gas is what lifts the dough. Home cooks lean on its gentle abrasive quality as well, scrubbing away residue without scratching.
Restaurants and busy households alike brush vegetables with a baking soda scrub to lift away wax or dirt, making preparation nicer and cleaner. It even steps into odor control. Open a box in the fridge, and those funky smells from leftovers seem less aggressive.
Clean-Up Crew Inside and Out
Cleaning up around the house, sodium bicarbonate becomes a go-to for me and many others tackling dirty sinks or greasy stovetops. It reacts with grime, loosening it just enough to wipe away, and you don’t have to worry about harsh chemical fumes. Many families trust it for brushing teeth, either on its own or in a pinch, though dentists recommend using it gently because rough powders can wear away enamel.
Health and Medicine Cabinet
Doctors and pharmacists also trust this compound. People use it as an antacid to settle a queasy stomach or heartburn. Hospitals mix it into IV solutions to correct certain blood chemistry imbalances, a practice trusted by medical professionals. Athletes sometimes use it for muscle recovery, though more research could nail down exactly how much it helps.
Making Life Easier in Agriculture
Gardeners and farmers count on sodium bicarbonate to help manage fungus on plant leaves. Sprinkling it around can cut down on powdery mildew, a pesky blight that ruins crops. I’ve talked to small-scale growers who swear by it as a budget-friendly plant defender.
Industrial and Emergency Applications
On a bigger scale, factories turn to this compound for smoothing out pH in pools and water treatment plants. Without a steady pH balance, metal pipes corrode or scale builds up fast. Its fire-extinguishing power steps in during emergencies too. Old-school fire extinguishers rely on sodium bicarbonate’s rapid breakdown under heat to choke off flames, especially in kitchen fires.
Thinking Critically About Safety
Most of the time, using this powder stays low-risk, but higher amounts—or careless industrial disposal—pose problems. Water sources get disrupted, and aquatic life suffers if big doses drift downstream. The Environmental Protection Agency and similar groups keep an eye on how much gets into waste streams.
Better Solutions by Learning from Experience
With so many applications, there’s no denying this compound saves time and headaches, but responsible handling goes hand in hand with all its benefits. I’ve learned through hands-on jobs and research that a little education pays off. Clear labeling and community guides help folks use this substance efficiently and keep the environment in mind. If we put effort into spreading knowledge, both at home and at work, there’s a path toward balancing usefulness with safety.
Checking Purity Isn’t Just a Box to Tick
Anyone who’s ever opened a container in a lab knows a number on a label isn’t just a detail to skip over. Purity shapes nearly everything about how a substance behaves and what it’s good for. Take pharmaceutical work—tiny changes in purity can shift results and raise red flags. In food or supplement production, unlisted impurities bring real worries about health and trust. I’ve seen whole batches recalled because a product didn’t hit its labeled purity level. A product reporting 99.9% should actually mean exactly that. Falsely labeled ingredients carry both legal and ethical risks, with contamination sometimes proving invisible until traced through testing.
Some work can manage with lower-grade material for early prototypes or basic reactions. But reproducibility and safety demand analytical data and batch certificates. Without those, guessing purity puts everyone’s reputation on the line. As much as we want to cut corners to save time, skipping verification piles up risk. Big names lost trust because of lapses in verifying what actually sat in their containers.
Storage Conditions: The Overlooked Step That Makes or Breaks a Product
Throwing just any compound on a shelf never works out well. From temperature shifts to light exposure, environment matters. At home, think of what happens when you leave strawberries out versus keeping them chilled—the changes aren’t subtle. Products degrade, byproducts form, and the end result doesn’t just differ in looks but in chemistry. Once, leaving a light-sensitive compound near a sunny window cost days of work. The solution came down to simple storage in amber bottles tucked in a refrigerator.
Water, too, is just as big a culprit as heat. Powders and reagents suck up moisture fast, clumping or even molding without warning. Even tiny amounts can ruin compounds meant for dry conditions. It sounds fussy to someone outside the field, but I’ve seen clients frustrated by variability caused by improper handling. Desiccators and controlled humidity rooms provide protection, but only if staff actually use them. In busy environments, shortcuts slip in and trouble eventually shows up in the data.
Real-World Storage: More Than Just a Written Protocol
Good storage practices start with labeling and end with daily habits. Anything meant for use needs clear signage for temperature and expiry. Putting everything in the fridge sounds easy, but not all chemicals play well with cold—some solids crash out, suspensions separate, and emulsions break down. Following safety data sheets for every chemical isn’t bureaucracy, it’s prevention. I’ve watched teams debate which fridge to use only to realize cross-contamination destroyed both samples and controls.
Companies have turned to barcode tracking and inventory management to stop things from slipping through the cracks. It’s not fail-proof, but these systems give visual reminders—alarms for expiring reagents or reminders to rotate stock. Staff training does more than keep regulations happy; it helps catch mistakes before they turn costly. Sharing clear SOPs and holding “storage audits” keep everyone sharp.
Taking Ownership
Understanding and respecting purity and storage isn’t just technical work. It builds confidence that what’s delivered today works as promised every time. Cutting corners in either area invites recalls, lost business, or worse, harm to others. Whether handling active pharmaceutical ingredients or specialty chemicals, the devil is in the details. Trust grows one batch and one carefully labeled vial at a time.
Respecting Chemical Risks
Every bottle or drum in a lab or plant tells a story about the risks it brings. Experience with tough chemicals teaches respect. In college, a splash of strong acid across a workbench sent everyone moving fast — not out of fear, but for respect of what just a drop could do to skin. Chemicals don’t listen to apologies. They follow the laws of nature, and if your hands are bare or your eyes uncovered, the consequences land in seconds.
Understanding Labels and Data Sheets
Before even opening a container, look at the label and reach for the safety data sheet — not as paperwork, but as protection. That sheet gives real insight: what burns, what fumes linger in the air, and what helps if things go wrong. Bleach might seem harmless since you find it at home, but in a concentrated form it destroys fabric and skin in a flash. Ammonia’s sharpness stings the eyes and lungs even before contact. Without that background, you might lean in too close or mix things without knowing the dangers.
Simple Gear, Big Difference
Chemicals don’t play favorites, no matter who handles them. Gloves are not all the same — latex weakens with strong solvents; nitrile holds up better. Goggles never go out of style, blocking splashes from reaching eyes that don’t heal so easily. Lab coats and aprons keep chemicals off shirts and skin. Closed shoes, no sandals no matter how hot it gets. Friends who forgot that pay for it with painful burns or ruined shoes.
Ventilation and Storage
My first job had a windowless stockroom. After a few days, headaches and coughs showed up. Underventilated spaces let toxic vapors collect, building up fast. Fume hoods and open windows save lungs and maybe lives, especially with volatile liquids like acetone or ammonia. Proper storage matters, too: strong acids away from bases, oxidizers far from anything that burns. Storage errors cause surprise reactions — pressure build-up, or even small explosions.
Handling Spills and Emergencies
One spill changes how you look at a crowded bench. Keeping spill kits handy is more than regulation; I’ve torn open powder bags and caught a cloud of caustic dust. Quick action — wiping up while wearing gloves, keeping a breathing mask nearby — turns panic into problem-solving. Everyone remembers the closest eye-wash and shower stations, and rehearses how to reach them. Trying to remember their location while chemicals burn your eyes leads nowhere good.
Never Work Alone
In big labs or small, never work with hazardous chemicals alone. If something goes wrong alone, a delayed response hurts more. One friend dropped a glass bottle of solvent; the sliced hand mattered less than the spreading fumes. A coworker stepped in, bandaged and led her out fast. Working together is an unspoken rule built from seeing rare accidents go sideways.
Continuous Learning
Over the years, safety habits form muscle memory, but complacency creeps in. Workshops, reminders taped to the wall, even quick refreshers at shift changes keep danger real. Technology keeps changing how chemicals interact and how they should be stored or handled. A culture of speaking up helps everyone stay sharp — it’s better to catch a small mistake than fix a big accident later.
Looking Past the Catalog Description
People spend hours comparing chemical products before they commit to buying. That’s not just about price or brand, but about hard data—numbers and structures that show what they’re really getting. Analytical tools such as NMR (nuclear magnetic resonance) and MS (mass spectrometry) tell a deeper story. This data takes a vague promise (“99% pure”) and turns it into something real—a fingerprint of the chemical itself.
Trust Grows From Transparency
Trust doesn’t appear overnight. It grows with each transparent detail a supplier shares. I've made a habit of checking certificates and spectra every time I need a new reagent. Clear NMR and MS data cuts through the fog. Without them, you’re left hoping the bottle matches the label. With them, you see what’s inside, every peak and shift carving out proof of structure and purity.
Regulators and Researchers Expect Rigor
Chemical products often slip into applications where precision decides safety. A single error in compound identity can ruin a whole synthesis, or worse, introduce health risks. Regulatory agencies look for robust documentation. Research journals insist on original data, not just supplier claims. When a company provides full spectra, you know they've put in the effort to verify their material. This isn’t just paperwork; it’s about real-world consequences.
Errors Have a Price
Ignoring analytical data isn’t just a shortcut—it’s a risk with a hefty price. I remember ordering what I thought was a simple building block for a project. A week and a dozen failed reactions later, I got suspicious. I found a mismatch between the NMR in the product notes and the compound’s expected structure. That wasted time, funding, and faith in the supplier. For researchers, one wrong spectrum can topple months of planning.
Suppliers Step Up Their Game
Competition among suppliers can have a real upside. Vendors post full NMR and MS spectra, not just summary sheets with a few lines about purity. More companies upload actual data files, not just photos of printouts. Some even offer spectra on request within a business day. This trend doesn’t just protect scientists; it weeds out vendors who cut corners. With the right info, researchers and industry teams make better calls, avoid bad batches, and move ahead with confidence.
Moving Toward Better Solutions
Every stakeholder has a stake in reliable analytical reporting. If manufacturers post current spectra for each lot, labs can check if the product fits their needs in real time. Databases that collect public analytical data make comparisons easier and mistakes rarer. Open sharing of this information still faces challenges, from cost to data security, but the push toward openness saves everyone headaches. Insisting on NMR and MS isn’t just fussiness. It sets a standard that keeps projects running, funds safe, and people healthy.