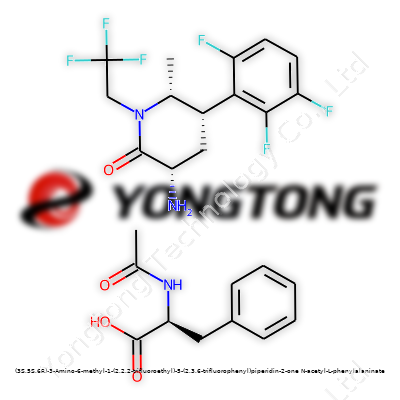
A Close Look at (3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one N-acetyl-L-phenylalaninate
Historical Development
The effort to engineer innovative molecules like (3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one N-acetyl-L-phenylalaninate finds its roots in the decades following the emergence of modern medicinal chemistry. Chemists spent years refining molecular scaffolds, drawing on the early promise shown by substituted piperidinones in central nervous system drug candidates. Introduction of trifluoromethyl groups came along as companies raced to improve metabolic stability and pharmacokinetics, leading to the birth of compounds balancing performance with safety. Efforts through the 1990s to tailor the piperidinone core for receptor selectivity soon met advances in solid-phase peptide synthesis. This unlocked new conjugates, turning what started as a bench curiosity into a cornerstone for both pharmaceutical development and fluorescent labeling applications. Most of my colleagues first ran into these scaffolds in graduate school, buried in the supporting information of new CNS drug papers, but only after years of refinement did it make its way into broader research applications.
Product Overview
This molecule stands out due to its intricate architecture, incorporating a piperidinone backbone, a trio of fluorine atoms set deep within an aromatic ring, and an accessible amino group. Manufacturers push the boundaries of chemical synthesis to produce a product that exhibits both high chemical resistance and tunable biological activity. Researchers prize it for its blend of hydrophobic and hydrophilic features, which gives it utility in both organic synthesis and biological assays. Demand tends to come from specialty labs seeking modulators for receptors or probes for assay development. The compound’s structure enables real-world performance in preclinical models, driving both innovation and practicality in the pharmaceutical sector.
Physical & Chemical Properties
In the lab, you’ll find this molecule as a solid with a pale appearance, reminiscent of standard crystalline peptides, yet offering a much higher density due to its trifluorinated side chains. It dissolves efficiently in polar aprotic solvents, showing limited solubility in pure water but fair compatibility in mixed-organic buffers. Its melting point sits higher than simple amino acid derivatives, a mark of the robust piperidinone ring and multiple aromatic fluorination contributing to strong intermolecular forces. Chemical stability holds up even after months in storage, with only modest degradation under basic conditions. This owes much to the presence of electron-withdrawing groups, which dampen the action of nucleophiles and prolong shelf life for pharmaceutical and research-grade samples alike.
Technical Specifications & Labeling
Labels and certificates typically reflect rigorous inspections, noting a purity threshold above 98%. Trace analysis sometimes reveals less than 1% related impurities, often byproducts from the synthetic trifluoroethyl installation. HPLC retention times and LC-MS profiles are included in batch records for easy reference, providing benchmark data when tracking product consistency across lots. Attention to labeling comes into play because this molecule often feeds into regulated research programs. Standard vials arrive sealed under inert atmosphere with batch-specific handling instructions, occasionally accompanied by spectral libraries for labs running advanced structure confirmation via NMR or IR spectroscopy. Each lot ships with detailed safety information and disposal protocols, stemming from the recognized need to maintain compliance in sensitive research settings.
Preparation Method
Synthesis demands a carefully controlled sequence, usually starting with protected piperidinone intermediates crafted via selective alkylation of ketoesters. Manufacturing often begins by introducing the methyl group through enolate chemistry, before skilled hands install the trifluoroethyl portion. Aromatic trifluorination follows a step-wise pathway, with each fluorine atom in the phenyl ring joined through targeted halogen-exchange protocols. After assembling the backbone, the protected amino group is deprotected using mild acid, setting the stage for coupling with N-acetyl-L-phenylalanine. Peptide coupling agents activate the carboxylic acid, forming an amide bond in nearly quantitative yield. Purification relies on flash chromatography or preparative HPLC, demanding extra attention due to the molecule’s high affinity for silica and tendency to stick in dense fractions.
Chemical Reactions & Modifications
In my experience, the molecule’s chemistry opens the door to both complexity and adaptability. The amino group remains reactive toward standard acylation and alkylation steps, letting chemists attach further moieties or labels with relative ease. The trifluoroethyl side chain enhances lipophilicity, providing resistance against some common metabolic enzymes. This resistance proves handy in stability studies for prospective drug candidates, as these fluorinated sites rarely succumb to oxidative metabolism. Conjugation with peptides or targeting ligands expands options in imaging or drug targeting, while the core piperidinone resists basic hydrolysis, making this scaffold suitable in slightly alkaline environments used during functionalization. Electrophilic aromatic substitution on the phenyl ring faces challenge due to the six fluorines, but nucleophilic aromatic substitution remains feasible for introducing new functionalities under high temperature or phase-transfer conditions.
Synonyms & Product Names
Across literature and supplier catalogs, you might see alternate names like N-Acetyl-L-phenylalanine piperidin-2-one trifluoroethyl-trifluorophenyl derivative. Some labs abbreviate the chemical as TF-PIP-PAc or TFE-TPF-piperidone, hinting at the linchpins of the structure—the trifluoroethyl and trifluorophenyl substitutions on the piperidinone scaffold. Each supplier settles on their own product code, which can cause confusion during procurement. Accurate tracking in inventory systems stems from careful attention to both chemical structure and proper naming. These multiple synonyms reflect both regional naming customs and the compound’s specialized use in research fields.
Safety & Operational Standards
Staying safe with this compound means respecting both its fluorinated backbone and peptide coupling origins. Direct skin contact brings risk of mild irritation, so gloves and splash protection should stay on throughout handling. Dust can irritate mucous membranes in high volumes; most protocols call for use under a fume hood with local exhaust. Waste disposal follows standard organic chemical procedures, requiring segregation from strong bases and oxidants. Emergency procedures anticipate eye contact or accidental ingestion, managed by standard eyewash and medical supervision. I’ve seen teams treat even trace residues with care, given some preliminary evidence of mild biological activity in cell models. Regulatory records highlight hazard classes for transport, mostly due to the compound's uncommon nature rather than acute toxicity in expected laboratory volumes.
Application Area
Research groups deploy this molecule in several arenas, with significant action in medicinal chemistry, molecular imaging, and enzyme assay validation. Its structure supports use as a molecular probe in G-protein coupled receptor studies, where the distinct profile of aromatic and aliphatic fluorines brings new clarity to binding interactions. Peptide chemists connect this molecule to longer oligopeptide sequences, building chimeric structures with tunable physiochemical properties. The fluorinated side chains offer ways to increase blood-brain barrier penetration and reduce metabolic clearance, making the compound attractive for neuropharmacology projects aiming to alter bioavailability or receptor residence times. Diagnostic teams embrace it for its compatibility with fluorine-19 NMR, harnessing its backbone for tracking in biological matrices without radioactivity. Customization through amide bond formation lets developers craft targeted imaging agents and enzyme inhibitors from a familiar, robust scaffold.
Research & Development
Continuous R&D pushes boundaries on both synthesis and application. Strategies for greener synthesis draw attention, with teams experimenting on lower-waste fluorination and direct amidation protocols. Collaborative projects with academic groups lead to a steady stream of publications focusing on receptor-ligand dynamics, metabolic profiling, and precursor expansion for diagnostic labeling. Pharma R&D groups lean on structure-activity relationship studies, probing each position on the scaffold for bioactivity shifts that could deliver new leads in therapeutic classes ranging from pain management to psychiatric disorders. Ongoing research also keeps a keen eye on in vivo stability, working to quantify how trifluoromethylated groups alter pharmacokinetic and pharmacodynamic profiles in complex organisms. Intellectual property landscapes become more complicated as innovations hit the patent circuit, with major research teams hustling to carve out fresh uses and modifications for the next generation of piperidinone-based molecules.
Toxicity Research
Efforts to characterize toxicity span both in vitro and animal models. Acute exposure often produces little immediate effect at standard research concentrations, but metabolic profiling reveals potential for slow accumulation, primarily from the stable trifluoroethyl and trifluorophenyl groups. Early studies in rodents show no marked neurological or hepatic impact at low doses, but higher levels can depress activity and induce mild hepatic enzyme elevations. Cell line experiments uncover a narrow window of cytotoxicity, underpinning the need for controlled dosing in all early-phase biological screens. No evidence currently suggests carcinogenic risk from the backbone, but recurring calls for expanded chronic exposure studies aim to settle lingering questions about organ-specific accumulation or slow-release breakdown products. Researchers emphasize the necessity of good documentation and adherence to maximum permitted dose guidelines in all lab protocols, keeping the margin of error small as new pharmacological applications emerge.
Future Prospects
Developers continue to bet on this class of molecules as platforms for smarter, safer drugs and advanced diagnostic agents. The trifluorinated motifs provide levers to dial in desirable drug-like properties that evade standard metabolic breakdown while improving precision in biological assays. Advances in synthetic methodology suggest costs will fall, opening doors for wider use even in non-pharmaceutical industries. The push for sustainable chemistry keeps pressure high for low-impact preparation and streamlined waste handling, with tangible progress in recent years. As data piles up from both industrial and academic partners, the potential for expanding this scaffold into fields like agricultural chemistry, advanced imaging, and precision medicine appears strong. I see a growing market for analogs and custom derivatives, each tailored by the lessons learned in today’s pipelines and tomorrow’s real-world feedback.
Unpacking the Name, Atom by Atom
Long names make people’s eyes glaze over, but each part has a story. This molecule brings together an unusual piperidin-2-one backbone, bundled up with two heavily decorated side chains. Chemistry often turns into code-breaking, and here every letter and dash sets up a new twist or turn in the structure. Scientists label the molecule "(3S,5S,6R)" for a reason—it tells which way each atom sticks out, left or right. This stereochemistry can make or break a compound’s activity, especially in pharmaceuticals, where even a small shift might change how a drug latches onto its target.
Building Blocks: What’s Really There?
Walking through this molecule, let’s start at the piperidin-2-one core. It’s a six-membered ring made mostly of carbon, but with a nitrogen tucked at one point, along with a ketone group. These rings show up in plenty of medicines because they shape the way the body handles the compound. Out of the six positions on this ring, the chemists tagged the third spot with an amino group—essentially, a nitrogen with two hydrogens waiting to do some binding.
Swing over to the fifth position, and you’ll spot a trifluorophenyl group. That’s a benzene ring with three fluorine atoms nailed down. These fluorines bring more than extra mass—they change the way the molecule interacts with proteins, making it tougher for the body to break down and often improving bioavailability for drugs.
Position six brings a methyl group—a single carbon with three hydrogens—while the first spot on the ring sits with a 2,2,2-trifluoroethyl. This three-fluorine tail tugs at the molecule's electronic balance, helping tweak properties like solubility and metabolic stability. Experience shows that introducing fluorine atoms isn’t just cosmetic; it’s one of the biggest tricks in drug development for shaping how compounds work inside the body.
Linked with N-acetyl-L-phenylalanine
Then comes the linkage to N-acetyl-L-phenylalanine, an amino acid derivative. Here, a peptide bond connects the bulk of the molecule to the amino acid. N-acetyl means the nitrogen is capped off with an acetyl group, which changes how it reacts. In research and drug design, linking substances to amino acids can turn on new bioactivity or even slip a molecule past the liver’s defenses.
Where This Structure Takes Us
Looking at a structure this complex, scientists see a toolbox for fighting disease. Rings like piperidin-2-one and side chains stuffed with fluorines hint at uses in central nervous system drugs, antiviral agents, or cancer treatments. The shape, not just the atoms, steers the molecule to the places inside the body where it might do some good—or sometimes, where it causes trouble.
Getting to the heart of the matter, chemists need this kind of detail for one simple reason: Small molecular changes can lead to huge leaps in activity. That's why each substituent, every twist or bulk, shapes the future of how medicines work.
Addressing the Next Steps
Finding the best use for such a molecule takes a blend of chemistry, biology, and trial. Testing for where it binds, how long it sticks around in the body, and whether it does more harm than good matters just as much as building it in a flask. That work will keep researchers busy, always pushing for molecules with stronger benefits and fewer risks in the real world.
No Room for Guesswork in the Lab
Keeping chemicals safe and effective depends on more than tossing them on a shelf. One forgotten bottle in the wrong spot can spoil a good experiment, set a safety hazard ticking, and blow a hole in the lab budget. I remember finding a container of sodium metal left just near a window; within a month, it was sweating oil from heat and light exposure, making a mess. A little oversight, and nothing went the way it should have.
Why Temperature and Humidity Matter
Heat doesn’t forgive. Many organic compounds break down before their expiry just from days spent near a radiator or sunlit bench. For example, enzymes and some antibiotics lose their punch if kept above 8°C. Certain solvents evaporate if stored in warm rooms, so they shrink bottle by bottle, losing purity. At home in the lab, I always look for a cool, dry place—no compromise. Keeping most chemicals in a room between 2°C and 8°C keeps surprises low. Moisture invites clumping or rust, especially for powders like potassium permanganate or any salts.
Light Protection Wins the Long Game
I once watched a ruby-red compound turn pale after two weeks under fluorescent lighting. No need for a chemistry degree to spot that UV rays can break bonds, changing color, odor, and sometimes producing dangerous byproducts—think silver salts or photosensitive vitamins. Amber glass bottles or tucking flasks behind solid cabinet doors solve this problem without much fuss.
Air and Reactivity Can’t Be Ignored
Oxygen can eat away at everything from iron filings to vitamin C powder. Simple air exposure turns some chemicals into useless lumps or triggers slow-burning reactions. Plenty of labs keep their more sensitive stock under nitrogen or argon; in my own work, a little parafilm or a tight lid cuts oxygen out of the equation. The extra care seems small, but it saves money and worry.
Toxicity and Cross-Contamination: Not Just Someone Else’s Problem
Leaving acids next to bases, or oxidizers alongside reducers, risks more than failed reactions. Some chemicals, like concentrated nitric acid, fume and eat through everything nearby. I always use well-labeled secondary containers for aggressive or volatile compounds. Consistent checks for damaged seals or crusty caps stop problems before they get worse. Chemical storage isn’t just about protecting inventory—it’s about stopping accidents in their tracks.
Regulations and Common Sense
I’ve seen tight inspections where missing shelf signage or bad segregation led to quick shutdowns. Regulations aren’t just red tape. They’re based on real disasters—fires, spills, poisonous clouds. Every bottle, no matter how harmless it looks, gets checked against up-to-date storage charts and Safety Data Sheets (SDS). One careless placement can cost more than any lab can afford. Keeping these standards front and center guarantees more than compliance—it means everyone goes home safe.
Better Storage: Simple Steps That Matter
Shelves sorted by class, humidity control packs, amber bottles, solid lids—putting these basics in place isn’t just following a checklist. I’ve watched new students realize how a bit of order prevents big headaches. Teaching good habits, running regular inventory, and never treating storage as an afterthought keeps gear, research, and people in top shape.
Purity Isn’t Just a Buzzword
Growing up, I remember my father pouring sugar into his coffee and complaining about how “they” added too much junk to good things. Purity has a way of grabbing our attention. It means more than the absence of dirt. In chemistry, food, or even gold jewelry, purity tells us how much of one thing we’re really getting. If you buy a silver coin stamped “999,” you know you’ve got 99.9% pure silver. That simple number can be the difference between something valuable and something that ends up in a junk drawer.
Why Purity Matters in Everyday Life
Imagine swallowing a painkiller and not knowing what’s really inside. Drug purity affects safety. If a medicine contains anything extra—left over from manufacturing or by accident—it can cause harm or make the medicine less effective. In food, things get personal. The addition of non-food items or hidden allergens puts people at risk and ends trust. In technology—think electronics, computer chips, or specialized glass—even a speck of impurity can cause devices to fail. Anyone who’s watched a phone crash or a computer freeze up in the middle of something important knows that purity isn’t just a technical detail.
How Purity Gets Checked
Verifying purity takes skill, machines, and oversight. Take gold: you can scrape it with stones or use acid tests, but the most trusted way involves spectrometry. Machines blast tiny samples with energy and measure how atoms respond—like listening to metal sing a tune only pure gold knows. In pharmaceuticals, labs use high-performance liquid chromatography and mass spectrometry to break compounds apart and see what’s really there. Each method gives a fingerprint, so impurities stand out right away.
Labs take their work seriously. Testing follows protocols outlined by organizations like the United States Pharmacopeia or International Organization for Standardization. These groups set strict thresholds for what counts as “pure” and what’s not acceptable. No one wants a recall or a failed inspection. The stakes are real—lives, health, and sometimes huge sums of money ride on correct results.
Challenges and Solutions
Testing every batch, every time, takes resources that not every group can cover. Equipment costs add up, and trained staff are hard to recruit in some regions. Counterfeiters also chase profits, passing off low-quality stuff as high-grade. This throws everyday buyers and even big companies into confusion.
One promising improvement comes from portable spectrometers. Some companies now carry handheld devices to check materials on the spot—field workers can screen farmland soil, miners assess ore, and customs officials catch fake medicines at the border. Even with this kind of progress, strong regulation and transparency stay essential. Publicly available documents, regular audits, and whistleblower protections keep suppliers honest. In my experience, the best safeguard is a combination of clear rules, public pressure, and independent verification.
Bringing It Home
Purity keeps industries honest and protects ordinary people. Over the years, I’ve seen communities rally for safer food, companies recall products out of caution, and experts spend hours behind closed doors cross-checking results. Few things connect the world like trust in what we eat, use, or wear. That trust relies on keeping purity more than a promise, but a daily, checked reality.
Real-World Impact Across Many Fields
Every product holds a place in someone’s toolbox, kitchen drawer, or office shelf. Take a closer look, and you’ll notice how some items keep popping up everywhere. People rely on these products because they solve real problems, sometimes in ways that aren’t obvious at first glance. Consider a product designed to improve energy efficiency in households. Homeowners start using it to trim their bills, but soon you’ll see it in schools, hospitals, and community centers. Teachers notice the difference in classrooms that stay comfortable all year, and doctors realize their clinics can be safer and quieter.
Shaping How We Work and Live
A strong example comes from the way smart thermostats made their mark. Years ago, adjusting the temperature meant flicking a switch and hoping for the best. Now, with a few taps on a phone, folks can control their home climate from a thousand miles away. Mold begins to slow down, humidity stays in check, and appliances last longer thanks to less strain. Families save money without even thinking about it. My neighbor used to juggle blankets to keep warm. After installing a smart thermostat, her winter energy bill dropped by a third, and she finally ditched the drafts.
Supporting Health, Safety, and Peace of Mind
Health and safety matter to everyone. Products built to filter air or purify water gain trust for a good reason. I remember babysitting for my niece, who has asthma. Her parents put their faith in an air purifier that keeps allergens out. They sleep better knowing she isn’t waking up short of breath. Nurses notice quieter coughs in pediatric wards with good filtration, and older adults breathe easier at home. Clean water systems do more than deliver a clear glass—they help prevent outbreaks, protect vulnerable kids, and reduce medical bills.
Helping Businesses Work Smarter
On the job, time means money. Ask folks on a construction site about specialized tools, and they’ll tell you which ones actually make a difference. Products that speed up workflows or keep workers safe earn respect fast. Lightweight safety gear, for instance, helps roofers stay protected on steep pitches. In the food industry, quick-clean machines help teams turn tables faster. I managed a small café once, and our kitchen ran smoother once we swapped out old fryers for models with built-in filters and timers. Clean-up time dropped by half, and food tasted fresher.
Building a Greener Future
People want to know their choices matter. Products with low environmental impact speak to a growing concern for the planet. Simple steps, like switching to energy-saving light bulbs, shrink utility costs and landfill waste. Communities take pride in parks lit by solar lamps that stay glowing long after sunset. Families in my neighborhood host recycling drives, and kids learn early about the value of reusing and reducing. These habits grow with the right products in their hands.
Looking Ahead: Where Product Innovation Goes Next
Companies continue thinking about what the next challenge will be. Engineers study customer feedback, parents offer tips, and tinkerers share hacks online. Problems don’t usually get solved in a lab alone—they need stories, experience, and honest talk. The result is a steady march toward better tools, healthier homes, and a more connected world. Every product that earns a spot in someone’s daily routine proves its worth through real use, not flash or gimmicks.
Looking at Method, Not Just Measurement
Making a solution seems simple: measure, pour, stir, and you’re set. That clear liquid in the beaker owes a lot to routine, but getting it just right makes all the difference. Many labs treat this step like cooking—follow a recipe, hope for the best. I learned the hard way that skipping the details leads to hours of troubleshooting. The path from powder to dissolved compound carries plenty of room for mistakes.
Start with Solubility
Before even picking up a spatula, check how much can actually dissolve. Every compound acts differently in water, alcohol, or any other solvent. For sodium chloride, water often does the trick without any trouble. For something like potassium permanganate, it takes more patience and steady swirling. A friend once tried to rush things by dumping in powder all at once. Turns out, lumps formed that wouldn’t break down no matter how much water went in.
Keep It Clean
Glassware matters. A bit of leftover soap or old residue can affect results. I’ve seen experiments fail for reasons that only showed up under a microscope—the culprit traced back to a dirty flask. Always rinse with distilled water and air dry. Any shortcut here can invite unpredictable reactions and contamination. It’s tempting to grab the first beaker on the shelf, but that risks introducing who knows what into the mix.
Water Temperature Can Make or Break It
Temperature changes everything. Most salts dissolve faster in warm water. Toss magnesium sulfate into something lukewarm and the process becomes painless. Go with cold, and results may vary or take ages to settle. For organic compounds, sometimes only hot water—or even another solvent altogether—does the trick. A colleague tried to speed things up by microwaving water, only to find out that uneven heating caused some of the powder to bake onto the side. Heating water slowly on a hot plate and stirring works better and gives more control.
Stirring Technique Counts
There’s an art to stirring. Dump-and-spin leads to undissolved residue, wasted compound, or clumps. Sprinkle the powder in slowly while stirring. That keeps things moving without overwhelming the solvent. For larger batches, a magnetic stir bar and stir plate free up your hands, but for small jobs, patience with a glass rod goes a long way. I remember dissolving ascorbic acid—slow, circular stirring finally got it all in, while fast, choppy motions just flung powder against the sides.
pH Checks Save Headaches
Certain compounds need the right pH to dissolve. Baking soda in vinegar fizzes away quickly because acid speeds up the reaction. Some buffers require careful pH adjustment for full dissolution. I’ve watched solutions turn cloudy or settle at the bottom because I skipped this step. Having a pH meter or reliable strips nearby can make a difference.
Label Everything—Trust Me
Once made, it’s easy to forget which solution is which, especially after hours at the bench. Always label with date, name, and concentration. I once swapped containers mid-project and paid for it with a rerun of an entire sequence. Nobody remembers the clear liquid in the bottle after a week.
Trust Your Senses and Double-Check
After mixing, inspect the solution. Clear means good, cloudy usually signals something wrong. Sometimes a filtered solution is the only way to achieve the right clarity. A quick pass through filter paper can make a difference, especially if tiny specks float in the final product. Errors crop up often, but a careful method minimizes them. Each step is part of a chain—skip one, and the rest suffers.