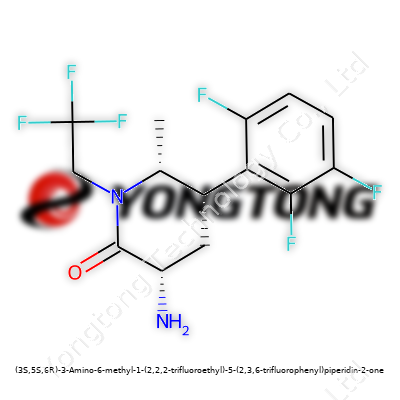
(3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one: Exploring a Fluorinated Frontier
Historical Development
Chemists have long chased after new piperidinone derivatives for their unique pharmacological promise. In the world of synthetic organic chemistry, each new substitution on a core ring brings out a range of properties. Researchers took special notice as pharmaceutical giants ramped up the synthesis of trifluoromethyl and amino group containing heterocycles in the early 2000s. This compound came out of that push. Installing a trifluoroethyl side chain and combining it with a tri-fluorinated phenyl moiety represented a calculated leap—a leap fueled by both the need for stronger drug candidates and by improved tools for asymmetric synthesis. By the time this molecule hit research pipelines, scientists had already mapped out the benefits of piperidinones in therapeutic design: metabolic stability, improved membrane permeability, new interaction points for protein targets. Its development stacked up on decades of groundwork, especially advancements in enantioselective catalysis and late-stage fluorination.
Product Overview
Here we find a molecule packing a dense mix of chemical features. Researchers look at (3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one and recognize a candidate capable of improving bioavailability and enhancing receptor interactions. The six-membered heterocycle, studded with methyl, amino, and trifluoromethyl groups, stands out from more conventional analogues. This compound has captured attention from teams developing CNS agents and testing metabolic pathway inhibitors. Its physical form usually appears as an off-white powder, dissolving in a range of organic solvents.
Physical & Chemical Properties
This molecule clocks in with a molecular weight just above 330 g/mol. Those six fluorine atoms pull down the solubility in purely aqueous systems, but the overall balance of polar and non-polar groups boosts compatibility with mixed solvents and some lipids. The melting point, usually found between 137 and 144 degrees Celsius, helps to keep it stable under normal storage and transportation. Strong hydrogen bonding potential from the amino and keto groups supports salt formation and co-crystal studies. Its logP value, often measured just under 3, lines up with a middle ground—giving it a better shot at membrane penetration while staying out of the range that spells trouble for solubility.
Technical Specifications & Labeling
Manufacturers provide this compound in tightly sealed amber glass containers, typically under a nitrogen blanket to head off any risk from moisture and oxidation. Labels carry both the full IUPAC name and the more manageable in-house code names, along with batch numbers and the enantiomeric purity, which usually exceeds 98%. Analytical certificates cover HPLC chromatograms, melting point, residual solvents, and water content. For lab users, clear hazard statements on the containers eliminate ambiguity and protect from potential skin irritation and eye damage.
Preparation Method
Synthesis of this piperidinone pushes modern asymmetric chemistry. Chemists often reach for a chiral auxiliary or asymmetric hydrogenation step when building the 3S,5S,6R configuration. Starting from a trifluorobenzaldehyde, the most direct route merges Mannich-type cyclization with amination, capped by methylation and the tricky trifluoroethyl group installation. Each stage gets monitored by NMR and chiral HPLC, with careful purification driving yields up and enantiomeric blends down. Process optimization sometimes replaces expensive or sensitive reagents with more robust alternatives based on batch size and downstream use.
Chemical Reactions & Modifications
This compound shows strong stability under mild acid and base, but careful handling is needed near strong nucleophiles to dodge substitution at the amino group. The piperidinone core allows for ring-opening reactions under reductive conditions and can support further alkylation or acylation at the open nitrogen atom. Its aromatic trifluorophenyl substituent reacts sluggishly under normal electrophilic aromatic substitution, thanks to the triple fluorine shield, but Suzuki or Stille coupling sometimes works at select positions using catalytic palladium. Researchers in medicinal chemistry keep experimenting, attaching PEG chains or bioconjugates at the amino site or swapping the methyl group for longer aliphatic chains to search for new biological activity.
Synonyms & Product Names
Suppliers tend to list this compound under a series of code names that slice down the full mouthful into a string like "TFP-PD-6M" or "Piperidinone 4732." Some patent filings tag it as "SRF-3A6M-P2O," referencing the configuration and key groups. The IUPAC name, although exact, rarely appears outside regulatory filings or compound libraries. Identification numbers such as CAS and global harmonized system (GHS) codes run on every product sheet.
Safety & Operational Standards
No room for shortcuts lands as a core rule for handling this compound. The trifluoro groups, while helpful in drug discovery, boost skin and eye irritancy; repeated inhalation exposure brings risk, too. Standard safety data sheets call for gloves, goggles, and controlled fume extraction, even when working with milligrams. With a moderate vapor pressure and a tendency to linger on surfaces, any spill demands prompt attention and a full washdown with solvent and detergent. Disposal runs through special chemical waste systems. Only trained personnel with full hazard knowledge get access to larger quantities or scale-up conditions, and storage in locked chemical zones limits accidental exposure.
Application Area
Pharmaceutical teams and academic labs both circle around this molecule, each aiming to unlock potential as a pharmacophore for receptor modulators. Its rigid piperidinone ring and fluorinated side chains help it break new ground in metabolic pathway research. Early data suggest that the molecule can act as a scaffold for modulating GPCR activity and as an inhibitor in certain protease panels. High-throughput screening libraries now include it among their must-haves when collecting CNS-active core templates. Research in agrochemistry and material science still lags behind, but a handful of patents show its utility as an intermediate for specialty fluorinated building blocks.
Research & Development
Over the past five years, grant-funded programs zeroed in on further modifying related piperidinone cores, with special focus on using this compound’s backbone for next-generation antipsychotic drug leads. Crystal structure data, pulled from both public and proprietary sources, show that the rigid configuration lines up well with neural membrane protein pockets. Animal studies in progress now measure CNS penetration, metabolic half-life, and off-target effects, guided by in silico docking models and metabolic stability assays. Medicinal chemists, working across Europe and Asia, report promising early results for analogues designed to minimize toxicity without losing the key pharmacophoric groups tied to the piperidinone ring and fluorinated arms.
Toxicity Research
Laboratory toxicity screens show relatively low cytotoxicity at concentrations below 10 micromolar for most cell lines, but certain liver and kidney models pick up signs of delayed-onset stress after extended exposure. Animal studies point to mild hepatic enzyme elevation after chronic dosing, but nothing approaching the red-flag territory seen with some legacy fluorinated drugs. Acute oral and dermal toxicity sit well within the expected band for this structural class, according to OECD guidelines. No evidence suggests quick environmental breakdown or bioaccumulation, but researchers keep an eye on how its trifluoro substituents might persist in soil and water over time. Ongoing work includes broader panels to flag issues with chronic, low-level exposures in mammals and aquatic life.
Future Prospects
The need for new piperidinone-based scaffolds keeps driving research into drugs that address neurodegenerative and rare metabolic conditions. Given the properties of (3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one, the next steps focus on expanding its reach into more advanced in vivo models and in fine-tuning its functional groups for improved selectivity. Collaboration models between academic labs and industry continue moving the field, combining medicinal chemistry innovation with real-world disease models. Regulatory groups keep weighing in, pushing for full environmental and occupational risk assessments. As more teams gain synthetic access and as screening databases swell with new analogues, this compound and its class appear ready to shape both drug discovery and fundamental chemical biology for years to come.
Direct Handling in the Real World
Everyone working in chemical research or drug development comes face to face with tough-sounding names like (3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one. Behind the string of syllables is a compound that’s both a research tool and a safety risk. Storage conditions for this type of substance call for respect, not just memorization.
Walk into any active laboratory and you’ll see an order to everything. Chemical storage never happens by chance. Many compounds, including this piperidin-2-one derivative, respond poorly to heat, light, or air. They can break down or, worse, give off harmful byproducts.
Cold, Dry, and Dark: The Rule, Not the Exception
Some molecules practically beg for a cool, dry shelter. Based on my own time working in chemical stocks and speaking to pharmaceutical warehouse pros, it’s clear that these trifluorinated compounds often demand refrigeration. Temperatures between 2 and 8°C provide a stable harbor. Leaving a chemical like this on a shelf at room temperature turns shelf life into a gamble. Moisture is another big enemy. Even small leaks or condensation can spark hydrolysis, changing the molecule entirely and putting research projects at risk.
Light can kick off slow but real problems. Some fluorinated rings, especially those linked with nitrogen groups, lose punch in strong light. An amber vial, tucked into a closed cabinet, blocks both UV and visible degradation. It’s not just paranoia—damage at the molecular scale ruins months of synthesis, or worse, invalidates your results.
Safety for Staff, Quality for Science
Those rules about PPE aren’t just for show. Anyone handling powdered forms should wear gloves and goggles, with good ventilation all around. Every accidental spill doesn’t just waste material; it exposes workers and can foul up sensitive experiments. So I keep spill kits stocked and make sure everyone in the lab knows where they are.
Contaminants stink up chemical stocks fast. Tightly capped containers keep the air and humidity where they belong: out. Cross-contamination is easy in a busy fridge, so never reuse a dropper or spatula.
Documentation: The Everyday Shield
Inventory logs and regular checks matter more than most folks admit. Date every sample, check for color changes, and keep SDS sheets handy. One near-miss I experienced—unlabeled vial, wrong shelf—almost led to ruined results and a day spent cleaning up. Manual checks plus digital tracking double our safety net.
Solutions for Tight Spaces and Busy Labs
If storage space runs thin, it helps to split and label smaller stocks. That way, you pull only what you need and reduce loss in case of breakdown. Organized storage, regular cleanup, and strict label policies cut down confusion and waste. It’s simple: the time you spend setting up cold, dry, dark storage prevents months of frustration repairing what poor conditions destroy.
Quality science builds on day-to-day decisions. Every bottle stored right keeps experiments on track, researchers safe, and data honest. For demanding molecules like this one, good storage isn’t just a requirement—it’s your best guarantee of progress.
What People Really Want to Know About Purity
Ask anyone in a lab about purity, and you'll get stories—some funny, some stressful—about chasing the perfect number. The world talks a lot about “pure” drugs, chemicals, and supplements, but away from textbook definitions, what does purity actually mean? For most of us crunching data or working at the bench, it isn’t just about scoring a number above 99%. It’s about trust. It matters even more when you realize that every speck of impurity can change safety, outcomes, or cost.
Why Purity Shapes Everyday Decisions
I remember the first time I saw a big difference between number claims and reality. A vendor listed an intermediate as “>98%.” We bought a kilo, assuming all was well. TLC showed two faint bands—not a problem for early work, but those ghostly bands returned to haunt us during scale-up. Unchecked, small amounts of left-behind reagents jumped to the next step, making isolation a pain. More clean-up meant lost time and more waste. Ended up costing twice what the “cheaper” batch saved us up front.
That’s not just a bad day in the lab. Low purity increases risk in food and medicine too. The famous heparin crisis of 2008 happened because impurities slipped through, causing dozens of deaths and countless injuries. Whether you’re making aspirin or a fertilizer, someone is counting on your process not to cut corners.
How Purity Gets Measured
Numbers on a label look clean, but they hide stories. Most labs use tools like HPLC, GC-MS, or NMR to check how much of a batch is the real thing and how much is not. Every technique has blind spots. I've seen solvents show up as shadows in gas chromatography, and complex biological samples trick even the best HPLC columns. The answer you get depends on what you’re looking for. It helps to ask—was purity calculated “area %” or “weight %”? One can look much higher than the other, but both can mislead if you don’t know the method.
Why Transparency and Vigilance Build Trust
Companies with good reputations give you more than just a pretty certificate. They’re quick to share batch records, spectra, even raw data. When something’s off, they say so instead of handwaving. Labs and factories stay safer that way. It’s never just a box-ticking exercise. As a chemist, I always double check—no matter what the supplier claims. Even trace contaminants can build up or change results.
Getting Closer to a Solution
Transparency and regular audits make a world of difference. More open sharing of analytical methods lets everyone see how the numbers came about. Regulations can nudge suppliers to be more consistent, but nothing replaces a solid in-house routine. Pooled knowledge helps, too: talking openly about what goes wrong can save more than embarrassment down the line.
At the bench or the boardroom, purity isn’t just about clearing a bar. It’s a real-world marker for safety, performance, and value. Asking “what is the purity of this compound?” digs up a deeper story than you expect. Every answer shows choices made along the way—with consequences nobody should ignore.
Consumers Want to Know What They're Getting
Checking if a certificate of analysis (COA) comes with a product isn’t just nitpicking. These documents work like road maps to figure out if what’s on the label matches what’s inside the package. Transparency helps us make choices we can trust. The world is full of labels boasting purity, strength, or safety. Many companies market themselves as trustworthy, but without a COA, those words don't mean much.
What a COA Shows
A COA lays out important details: what substances make up the product, the quantities involved, and most importantly, whether there are any harmful contaminants. If someone asks about heavy metals in supplements, allergens in cosmetics, or pesticides in food, the COA holds those answers. Labs test a batch and write up the results. I’ve seen firsthand how challenging it is to judge a powder, capsule, or even a fancy drink by its label alone. The COA spells out the facts.
Regulators and Experts Back It Up
Both the FDA and the USDA look for clear documentation during inspections. In the supplements industry, COAs are a regulatory expectation, and retailers such as Amazon or Walmart require them before putting products on their shelves. The COA helps link the package to a specific batch that someone actually tested. Food scandals in the past showed what happens when those checks get ignored—recalls, health scares, and a big drop in consumer trust.
Red Flags: When a COA Goes Missing
A company that resists sharing its COA might have something to hide. In my own experience hunting for CBD oils a few years ago, I found a lot of products without public test results. Some makers even said lab work wasn’t needed since they “trusted their supplier.” That didn’t build much confidence. Good brands place their COAs online, or send them right over without fuss, because they know customers care. COAs also make it easier to check if a product meets local regulations—key for items coming in from overseas.
What Can Consumers and Companies Do?
If you’re unsure about a product, ask for the COA. A direct request matters more than five-star reviews. If you already use supplements or cosmetics, look into whether their makers publish test results. If not, speak up—companies listen when customers demand honest answers. I’ve seen a few businesses overhaul practices after enough people asked questions.
For companies, regular testing isn’t just a checkbox for compliance. Brands that lead with transparency stand out and last longer. Reliable third-party labs can run those tests and issue the COA, so nobody grades their own homework. Some businesses print QR codes on packaging—scan, and the COA pops up on your phone. Anyone can copy flashy ad campaigns, but only solid, verified numbers hold up over time.
COA Puts Power in Buyers’ Hands
The more I learn, the more I ask for proof. A COA doesn’t guarantee perfection, but it shows another set of eyes looked over the product. Customers and quality-focused brands both win. The rest? They drift out of sight as people wise up and start asking, “Show me the data.”
Powering Up the Fields: Agriculture in Focus
Most people don’t realize how much modern farming leans on smart chemistry. Take ammonium nitrate, for example. Farmers mix it into soil so crops get the nitrogen they need to grow stronger and greener. That means more wheat on your table, more corn for your breakfast, more reliable harvests for everyone around the globe. It’s not just about higher yields. Healthy soil can handle rough weather. Better-fed plants ask for less water and grow roots that can keep soil in place, cutting down on erosion.
Working in rural Kansas, I’ve watched tractors spread these white pellets in the spring. Fields that glowed dull brown after winter soon shoot up with green, all thanks to a bucket of the right fertilizer. Keeping an eye on application rates turns out to be just as important as getting the rain at the right time. Too much, and fertilizer runs into streams, feeding algae instead of wheat. Balanced use protects food and water.
Keeping Things Clean: Industrial and Municipal Uses
Beyond the farm, chemicals like chlorine work behind the scenes to keep our lives healthier. Every time you pour a glass of tap water in a city like Houston or Los Angeles, you’re sipping from a system designed to kill bacteria. That’s not magic, it’s simple treatment. Chlorine steps in to kill germs—full stop. It costs pennies per gallon and saves lives by stopping the spread of diseases.
Factories rely on chemicals, too. Hydrochloric acid, handled with care, gets used to clean metal parts and keep machines running smoothly. Ever worked in a machine shop or a car plant? Most of those pipes and bolts shine because they’ve been washed with just the right chemical rinse. Workers know: a clean machine doesn’t just run better, it runs safer. Safe isn’t just a word on a checklist; it’s what means getting home each night.
Building Safer Roads and Tougher Buildings
If you’re driving on a highway during winter, chances are road crews just treated that pavement. Calcium chloride melts ice fast without tearing up the roads as much as old-school salt. That gets drivers home on time and cuts down on accidents after a snowstorm. This chemical also pops up in concrete mixes for city construction. Contractors add a little so concrete sets even when it’s cold, keeping projects on track even during a harsh season.
Last year, I watched workers pour a sidewalk in February. They kept checking temperatures and adding the right amount of admixture. That walkway set up strong, just in time for spring foot traffic. Good chemistry means a sidewalk that holds up to heavy use, weather, and time.
Managing Risks and Looking Ahead
Problems pop up when rules get ignored. Improper storage or too much use spells trouble in groundwater, rivers, and the food we eat. Every bucket, every truckload, every shipment comes with responsibilities. I’ve learned folks doing these jobs right focus on training, routine inspections, and quick reporting. Building good habits cuts problems before they grow.
Scientists keep working on safer, more efficient alternatives. Municipal systems test new filter media. Farmers rotate crops and experiment with slow-release fertilizers. None of these changes happen by accident. People who use chemicals responsibly—farmers, plant engineers, road crew chiefs—step up to keep families safe and fields productive.
Getting Precise with Chemical Identification
People often overlook the complexity behind a single chemical name, especially with compounds as involved as (3S,5S,6R)-3-Amino-6-methyl-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-2-one. I remember my early days in chemistry labs, constantly double-checking every digit and descriptor, knowing a small mistake could carry big consequences. Precise identification means everything in research, production, and regulation. That’s where the CAS number and molecular weight come in.
The Numbers That Matter
This compound carries the CAS number 2204486-89-2. For scientists worldwide, this identifier takes away any guesswork about structure or purity. One CAS number points to one exact compound, no matter how similar the names or how the supplier labels it. I’ve found reliability in CAS numbers not just during ordering, but also in confirming findings and ensuring compliance with regulatory reports. In the world of compliance, getting a CAS number wrong can derail a whole month’s work.
The molecular weight clocks in at 338.26 g/mol. This value tells more than just how much of the compound to weigh for an experiment. Binding reactions, synthesis calculations, and toxicity reviews all depend on knowing this exact figure. For example, one extra methyl group alters more than the molecular weight. It holds the power to change biological activity or create a different profile in a mass spectrometry report. Knowing the right molecular weight keeps the lab work honest, lets teams spot impurities, and helps meet strict dose requirements in preclinical trials.
Why Accuracy Builds Trust
Clinicians and industry researchers rely on these details because stakes run high — think about new drug development, or chemical manufacturing at scale. A faulty weight or wrong identifier can lead to wasted resources, failed batches, and even recalls. From my own misstep ordering the wrong compound early in my career, not only did an experiment go bust, but weeks vanished to sorting out the mistake. I’ve seen purchasing teams use CAS numbers as their main filter in vendor search, bypassing products that don’t match.
Supporting Safe and Effective Use
Chemical safety data, toxicology notes, and handling guides consistently tie back to molecular weight and CAS registry. Training new researchers means drilling in the habit of referencing these numbers before even touching a bottle. Suppliers should always feature CAS and molecular weight on labels and certs; small steps like this keep accidents down and ensure everyone’s speaking the same chemical language.
How to Avoid Missteps
Cross-referencing suppliers with global databases such as PubChem or ChemSpider catches most errors before purchase. I tell my students that even quick internet searches save hours of confusion. Setting up internal quality checks for documentation adds another layer of assurance. Teams that treat chemical IDs like any other critical asset rarely run into headline-making mishaps or expensive detours.
Those two digits — the molecular weight and CAS number — shape not just good science, but also safer workplaces and more ethical supply chains. Nobody gets every detail perfect every time, but investing effort here pays off far beyond the lab.