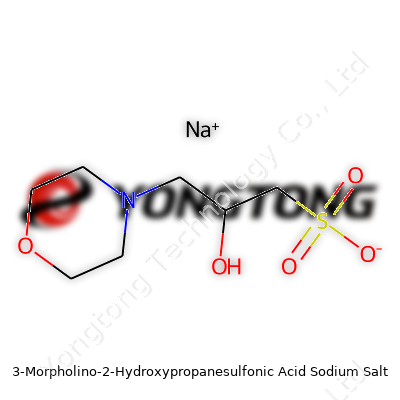
3-Morpholino-2-Hydroxypropanesulfonic Acid Sodium Salt: Exploring the Details
Historical Development
Once folks started looking beyond classic buffers, a niche opened up in biochemical research for substances with precise pH control that didn’t mess up sensitive assays. In the mid-20th century, scientists began to invent “Good’s buffers”—named after Norman Good—testing compounds with both ion stability and minimal biological reactivity. Among this group, 3-Morpholino-2-Hydroxypropanesulfonic Acid (commonly known as MOPSO) appeared, soon followed by its sodium salt. Labs wanted solutions that didn’t drift over time or react with enzymes and proteins. MOPSO picked up steam as researchers tired of the headaches older buffers caused. I remember in graduate school, folks would talk about how these new buffers were fixing data that once seemed impossible to clean up.
Product Overview
MOPSO sodium salt shows up as a refined, white or off-white powder. The product meets demands seen in life sciences, showing up in protein purification, cell biology, and diagnostic kit manufacturing. Its reputation centers on stable, reproducible pH—vital for experiments where even small shifts throw everything off. No surprise lots of biotech suppliers stock it. In one protein crystallization class, I watched as researchers compared buffer options. The choice often came down to whether a buffer introduced unwanted side effects. The instructor laid out several, and MOPSO sodium salt won out because it held pH without interfering with commonly used reagents.
Physical & Chemical Properties
Physical details guide technicians through their work: MOPSO sodium salt dissolves quickly in water, producing a clear, colorless solution. It melts at high temperatures, has low volatility, and remains chemically stable across the typical laboratory pH range. With a pKa around 6.9 at 25°C, and a working range between 6.5 and 7.9, it works well in applications mimicking physiological conditions. High water solubility (over 100 g/L at room temperature) ensures it mixes clean in most protocols. Chemists take careful note of its neutrality with respect to enzymatic systems; the molecule doesn’t easily form complexes with metal ions, avoiding the pitfalls some other buffers bring.
Technical Specifications & Labeling
Sourcing high-purity MOPSO sodium salt matters in clinical and research settings. Suppliers tend to report purity above 99%, with low moisture content and stringent limits on heavy metals and microbial contamination. High-performance liquid chromatography (HPLC) often provides the certificate of analysis backbone, with batch traceability baked into the labeling. Reputable distributors include precise molecular weight (255.26 g/mol for the sodium salt), identify batch numbers, shelf life (usually a couple of years if stored cool and dry), and ensure each container remains sealed. In my own lab experience, we’d always double-check every vial label against our inventory records before breaking the seal. Anything off about labeling was enough justification to send it back.
Preparation Method
Lab preparation typically involves reacting morpholine with epichlorohydrin under controlled conditions, producing an intermediate, followed by sulfonation with sodium sulfite. Each step needs careful temperature and pH control, with purification steps like filtration and crystallization defining quality. A typical process blends chemical engineering and careful analytical measurement, since residual precursors or side products can throw off sensitive experiments. Automation helps large-scale producers maintain batch consistency, but even bench-scale synthesis calls for solid glassware, precise heating, and reliable pH meters. My former research group built their own protocols, adapting published methods while troubleshooting yield and purity with each batch.
Chemical Reactions & Modifications
MOPSO sodium salt resists most unwanted chemical reactions in biological systems, which builds its appeal. The morpholino group and sulfonic acid present few reactive handles, so the buffer stands up to oxidizing and reducing environments. Still, it can undergo esterification or amidation under nonstandard lab conditions. Most users don’t tweak it, but some research groups modify it for special analytical detection or as part of affinity matrices. Chemically, it plays well in multicomponent reactions, rarely causing cross-talk or unpredictable interference—the reason why technicians in molecular biology labs often reach for it without a second thought.
Synonyms & Product Names
The chemical goes by several names depending on supplier and context, including MOPSO sodium salt, 3-morpholino-2-hydroxypropanesulfonic acid sodium salt, and occasionally just MOPSO-Na. Industry catalogs might list additional trade names, so keeping track across brands and batches becomes part of the job. As I rotated through different labs, every technician kept logbooks filled with the various labels this compound picked up from different suppliers; some standardized on one acronym to avoid confusion in shared protocols and records.
Safety & Operational Standards
Standard laboratory safety applies with MOPSO sodium salt—gloves, goggles, and dust controls during weighing or solution prep. Acute toxicity runs low, though repeated exposure can irritate eyes or mucous membranes. Spillage clean-up focuses on sweeping up powder before it enters drains or water supplies. Fire hazards rarely come into play, as the compound doesn’t ignite under normal handling conditions. Larger facilities typically include it on safety data sheets lodged with local authorities. Training sessions for new hires cover not just basic precautions, but also the need to track expiration dates to avoid questionable batches. I recall a lab audit where expired buffer stocks brought the biggest red flags, a reminder that operational standards aren’t just paperwork—they keep experiments and people safe.
Application Area
Biology labs everywhere rely on MOPSO sodium salt for buffer solutions in electrophoresis, enzyme assays, DNA/RNA extractions, and chromatography. Hospitals and diagnostics companies turn to it for consistency and nonreactivity in test strip formulation. It’s used as a stabilizer in vaccine formulations, and researchers studying protein folding run MOPSO buffers on auto-pilot, trusting that the buffer won’t shift pH mid-process. Environmental labs sometimes use it in controlled pH studies because it resists breakdown and photolysis. My mentors emphasized documenting every buffer used, since discovery often depends on pinpointing even the smallest experimental variable. MOPSO sodium salt earned trust because it fades well into the background, letting scientists see what really matters in their samples.
Research & Development
Academic groups continue to tinker with new buffering compounds, but MOPSO sodium salt keeps turning up in studies that test buffer effect on reaction rates, protein stability, and cell culture health. Researchers have documented its low interference profile with a range of enzymes and antibody-antigen interactions. Newer work has explored pairing MOPSO with microfluidic systems or integrating it into hydrogels for tissue engineering. Published papers still compare it to older buffers, often showing measurable improvements in stability and reaction clarity. Large research consortia sometimes ask for buffer performance summary sheets before launching collaborative studies. I remember sitting through review meetings where data quality rode on buffer selection, and people brought up MOPSO sodium salt as the gold standard for keeping experiments reproducible across partner labs.
Toxicity Research
So far, animal and cell culture studies back up MOPSO sodium salt’s safety at concentrations typical for lab work. Metabolism studies suggest the body doesn’t absorb or break it down easily, reducing risk in case of minor exposure. Acute and chronic toxicity assays flag low risk in mammals, though every compound can harm at high enough doses. Regulators haven’t identified long-term toxicity, but labs lock in standard practice to avoid surprises. Reviewing the literature feels reassuring, but many scientists argue for ongoing surveillance, since industrial scale-up can uncover unusual risks. My own experience with new chemicals always runs through a checklist: review the MSDS, consult recent studies, and share findings with the team before bringing new buffer stocks into the workflow.
Future Prospects
Looking ahead, MOPSO sodium salt’s future seems tied to ongoing demand for robust, low-interference buffers in both classic bench science and emerging biotech. More groups construct miniaturized assays that challenge older buffer choices, raising the value of reliable, inert options. Companies working in diagnostics and drug discovery lean toward compounds like MOPSO sodium salt because reproducibility drives everything from research success to regulatory approval. Environmental researchers investigating microplastic impacts or waterborne enzyme reactions turn to buffers that won’t interact with trace components. More labs digitize and automate their workflows, and dependable chemical stocks form the backbone of these advances. As someone who watched the move from hand-mixed buffer stocks to automated platforms, I see the continuing need for innovative chemistry backed by deep safety data and decades of troubleshooting.
Understanding Its Place in the Lab
Walk into any modern bioscience or chemistry lab and you’ll find more clear liquids and powders than you’ll ever see in a grocery store. Among those, buffers hold a special place, and 3-Morpholino-2-Hydroxypropanesulfonic Acid Sodium Salt—often called MOPSO-Na—gets noticed more often these days. In my time working through protein purification protocols, the importance of the right buffer stands out quickly. Getting pH wrong in an experiment might leave you with a week’s work lost. MOPSO-Na gives researchers a way to manage pH in ways that tools like phosphate buffers simply can’t match, especially around slightly acidic conditions.
This buffer compound shows up in biochemistry and molecular biology experiments, mostly due to its stable pH range, which runs from 6.2 to 7.6. Think of how often enzymes, proteins, or antibodies need a certain environment to behave properly. With the “Goldilocks” effect in biology—not too acidic, not too alkaline—MOPSO-Na hits that sweet spot. In practice, it helps keep experimental conditions steady, taking into account challenges like temperature shifts or the influence of additional reagents. The benefit of this kind of reliability can make all the difference in research where consistency is king. It’s hard to overstate how a failed experiment can turn on a wandering pH.
Strengths and Specific Applications
MOPSO-Na’s low interaction with biological molecules makes it a go-to for labs that can’t afford surprises. Non-specific binding can rip apart your signal-to-noise ratio, and years back, I learned the hard way that sometimes a cheap buffer means fuzzy data. MOPSO-Na resists binding to magnesium, calcium, or heavy metals—a key for enzymatic studies and nucleic acid work. Anyone following Michaelis-Menten kinetics, or looking for real signals in sensitive assays, wants to remove variables. Buffers like MOPSO-Na clear the slate.
A common issue in protein chemistry is preserving protein structure and activity throughout a process. During purification at the bench, exposure to temperature swings or lingering contaminants throws off measurements. I’ve seen cases where MOPSO-Na kept protein active, where others failed and left everything denatured. That matters in vaccine research or enzyme engineering—where each microgram counts and repeatability is everything.
Safety, Accessibility, and Environmental Considerations
The standard for MOPSO-Na salts sits at high purity. Any reliable supplier will publish a Certificate of Analysis, and reproducibility depends on manufacturers sticking to the script. Over time, demand from the research community has kept costs steady, making it accessible not just to large pharma, but also to smaller university labs.
There’s also an increasing pressure for labs to limit environmental footprint. Traditional buffers based on phosphate or TRIS have raised red flags over disposal and ecosystem impact. From conversations with colleagues, switching to Good’s buffers (like MOPSO-Na) addresses these worries. They tend to degrade without producing harmful byproducts that end up in the water supply or soil. Labs adopting sustainable practices look out for this, even if it means recalibrating protocols or retraining staff.
How Better Buffers Push Science Forward
It’s tempting to see buffers as background props, but issues like experimental reproducibility, lab safety, and environmental stewardship tie in closely. My own projects improved the day that buffer choice moved from afterthought to conscious strategy. MOPSO-Na’s rise tells a bigger story: reliable ingredients let scientists focus on discovery, not fixing yesterday’s mistakes. As bench research continues pushing boundaries, the right chemical tools allow attention to shift toward bigger questions, not managing ever-shifting pH readings.
Temperature Stands Front and Center
I’ve spent time behind lab benches and inside storage rooms, seeing what happens when guidelines get tossed aside. There’s a reason so many compounds carry strict temperature instructions. Heat and sunlight don’t just speed up spoilage; certain chemicals break down, clump, or even grow dangerous when not kept cool. Even something as simple as ascorbic acid will deteriorate quickly if left in a warm supply closet. So, compounds such as sensitive reagents, many pharmaceuticals, and even some everyday vitamin supplements, stay stable longest chilled in a fridge (2–8°C) or—rarely—frozen. Failing to follow those directions means wasted money and potential safety risks, especially for anything that goes into bodies or reacts in a test.
Moisture Brings Trouble
Once, I saw an entire batch of sodium borohydride ruined by a humid summer’s day. Moisture, even at low levels in the air, causes certain solids to cake up, shift color, or react just sitting on the shelf. Some powders suck in water from thin air, and suddenly, their chemical structure has changed. Silica gel packs or tightly sealed containers aren't just optional—they become real insurance against disaster. Even household pain relievers rely on dry storage for maximum shelf life. Ignoring those plain-seeming instructions causes waste that most folks and labs can’t afford.
Light Exposure Isn’t Just About Fading Labels
Walking through a storage room for the first time, I remember the surprise of finding bottles wrapped with aluminum foil. This wasn’t just about tradition. Compounds such as silver nitrate and some antibiotics break down in bright light, sometimes into toxic or useless remnants. That’s why amber bottles or foiled containers aren’t overkill—they block out harm and stretch out usable life. Ultraviolet rays speed up chemical changes in all sorts of compounds. I’ve seen clear glass bottles sitting on a windowsill lead to ruined stocks more than once. Keeping products in dark places makes a world of difference, and label instructions usually spell this out plainly.
Cleanliness Cuts Down on Cross-Contamination
Storing chemicals on dirty shelves with open lids always felt like asking for trouble. Dust, old spills, and poor organization lead to mistakes or contamination fast. Safe practices grow out of habits: cleaning equipment before storage, labeling clearly, keeping incompatible compounds separated. Simple rules—don’t return unused material to the original bottle, keep acids away from bases, never store volatile liquids near heat sources—can feel tedious but save lives and livelihoods.
Why Experience and Documentation Matter
Google’s E-E-A-T principles push everyone—professionals and hobbyists—to rely on credible sources and real experience. Using information from safety data sheets and manufacturer instructions doesn’t just check a box for compliance. They draw on decades of learning, error, and improvement. It's not about bureaucracy; it's about preserving resources and keeping people safe.
Rethinking Everyday Practice
Mistakes happen. More often than not, someone missed an update or shrugged off a storage warning. The fix comes down to training, labeling, and honest review of practices. Investing in proper storage cabinets, reliable refrigeration, and humidity control pays off quickly. Regular checks—the kind built into good lab routines—catch small problems before they snowball.
Key Takeaways from Real Labs
Getting storage conditions right helps keep compound integrity, protects budgets, and reduces health risks. Paying attention to temperature, moisture, light, and good housekeeping creates a foundation you can trust, whether you're running experiments or keeping medical supplies safe at home. No fancy vocabulary needed—just habits grounded in fact and common sense make all the difference.
Experience Counts in Lab Decisions
Every scientist has stood in front of a shelf, reading a label and asking the same question: is this really going to work with sensitive cells or tricky DNA? A product can carry every certification in the world, but real value shows up in the data and the story your experiments tell. Over years of work in labs, one truth stands out—results depend on the quality and traceability of what’s in your bottle, flask, or tube.
Purity Makes or Breaks Experiments
Let’s talk about purity. For cell culture, contamination can throw off months of work. Fungal or bacterial invaders sneak in unseen. For molecular biology, a single enzyme contaminant can block a PCR or sabotage a cloning experiment. Reputable products don’t just claim to be “molecular biology grade”—they show batch records, offer sterility testing, and prove their claims through certificates of analysis.
Components Shape Everything
With cell lines, even small changes in salt or vitamin content can make cells behave differently. Human stem cells are notorious for responding to subtle shifts in nutrient quality. I once switched brands of fetal bovine serum, and my cultures behaved completely differently. Cells grew more slowly and lost their characteristic shapes. Only after reading the full product documentation did I find out: the filtration process and final protein concentrations barely matched, despite identical catalog descriptions. It made clear just how carefully every component should be checked.
Stability and Lot Consistency
Experiment progress can vanish fast when products vary from lot to lot. For western blot reagents, I’ve seen blocked membranes light up beautifully one week and barely register the next with a new bottle. Scientists depend on suppliers who run regular stability studies and provide transparent test results for each lot. Some companies now share DS-CAM (Data, Safety and Certificate Assurance Management) links, offering scientists real insight into how lots performed under validation testing. Reliable suppliers openly list expiration dates and keep documentation easy to find.
Supporting Data and Real Tests
Suitability goes beyond a datasheet. I trust a supplier more if they share results from actual cell-based or DNA-based assays. Documentation should show sterility checks, mycoplasma testing for cell culture media, and nuclease-free assurance for molecular biology needs. Just packaging a product in a clean room doesn’t guarantee freedom from harmful enzymes or toxins. Users should ask for direct application data: images of healthy cells grown with the reagent, or clear amplification from PCR using only the supplied buffer. It’s surprising how few companies publish this kind of proof, but those that do win lasting trust.
Thinking Ahead: Solutions and Improvements
Labs can reduce risk by validating any new reagent alongside a trusted control. Running parallel cultures or reactions with a new lot and the old standby helps detect subtle changes. Collecting feedback and sharing it with suppliers creates an evidence loop, encouraging them to strengthen testing, transparency, and support. I’ve seen research groups set up custom contracts with suppliers to ensure a stable supply of reagent lots, lowering surprises and keeping projects on track.
Details matter. From purity and documented batch tests to vendor transparency and real-world assay data, there’s no shortcut. Each step safeguards scientific progress and trust in the process—a lesson learned time and again at the bench.
Chemistry Breaks Down Complex Problems, Not Just Molecules
People working in life sciences, buffers, or biochemistry often bump into names that feel more like tongue twisters than chemical compounds. One of those is 3-Morpholino-2-Hydroxypropanesulfonic Acid Sodium Salt, which goes by MOPSO-Na. The formula for this compound reads as C7H15NO6SNa. The molecular weight clocks in at about 281.25 g/mol. It seems dry on paper, but the number matters. Exact calculation supports proper dosing, experiment repeatability, and makes sure that what goes into a buffer recipe matches what researchers intended.
Why Getting Chemical Details Right Impacts Research Quality
Every molecular detail becomes a building block in research. My own run-ins with sloppy reagent prep have taught me that even one small calculation error can roll into big problems down the line— failed PCRs, inconsistent cell growth, or off-target reactions.
It's easy to think these calculations only matter for academic nitpicking, but accuracy keeps experiments honest. At the industrial or clinical level, mistakes don't just waste time; they tie up lab schedules, pile on costs, and, at worst, endanger patient samples. Companies like Sigma-Aldrich and Thermo Fisher Scientific put out detailed specs for good reason; they help people avoid headaches and keep science moving.
MOPSO-Na’s Role in Real-World Labs
People use MOPSO-Na mainly for making biological buffers. Its sulfonic acid group keeps pH stable, which is critical for any work involving enzymes, proteins, or nucleic acids. In my own benchtop work, buffers with unreliable pH cause more troubleshooting than almost anything else.
Commercial labs rely on buffers that don’t shift their chemistry over a few hours’ time. That keeps experiments fair and outcomes reproducible. The sodium salt form helps with solubility, handling, and compatibility with downstream assays. Some labs switch from older Good’s buffers to MOPSO-Na for those adjustments, particularly if they want less background signal in sensitive measurements.
Transparency, Traceability, and Making Solutions
Labs in regulated spaces can’t wing it with chemical identities. Every bottle that goes into a process needs its traceability. I’ve seen audits hinge on details as small as a wrong supplier catalog number or misplaced certificate of analysis. The exact chemical formula tells people what atoms are present, while the molecular weight tells them how to weigh it out. If the molar mass is off, a buffer prepped for 50 mM might land at 45 mM, and that drift alone can shift enzyme activity in a costly experiment.
Small Steps Toward Fewer Errors in the Lab
Science runs smoother when everyone spends a little more time double-checking key values—the right formula, the right molecular weight, and the right paperwork. Many lab disasters would never have happened if someone had paused for a minute to review the label. Digital lab notebooks and chemical databases, such as PubChem or ChemSpider, give current info that saves a lot of scrambling later.
Suppliers play a part, too. They can update product info, publish clear COAs, and regularly verify their data. At the end of the day, labs that train people to respect details—right down to the formula and weight—lose less time and produce science that stands up to scrutiny.
Grabbing Reliable Materials First
Every experiment rides on the back of good preparation. A lab product should never get tossed into solution straight out of the warehouse. Unpacking begins by checking the label and certificate of analysis. Experience says a lab notebook and a moment for double-checking numbers on the label guard against wasted effort later. Clean glassware, high-quality reagents, and the right water type protect against false results.
Understanding the Solvent’s Role
A quick glance at the product’s documentation will say if it works in water or if something stronger, like ethanol or DMSO, serves better. Attempting to dissolve something in plain tap water often brings more questions than answers. Years in the lab taught me that knowing the solubility curve can prevent a wasted day swirling an uncooperative suspension.
Weighing Out the Product
Accuracy remains king. Steady hands, an analytical balance, and a dry spatula become standard tools. Scooping too much invites headaches in calculations and dilutions. Many colleagues still print out calculation sheets for complex molarities, and there's no shame in that—errors compound fast. Measuring to the nearest milligram protects results, and any extras or clumps left on the weighing paper stay out of the container.
Mixing without Mishaps
Once the powder sits in the beaker, patience matters. Rushing leads to undissolved clumps or air bubbles, both of which leave concentrations off. Stirring rods and magnetic stirrers do more good than violent shaking. If heat helps, a temperature-controlled water bath prevents scorch marks or broken glass, but a stirring plate often does the trick for most salts and buffers. Don’t ignore the label about temperature limits—too much heat can destroy sensitive components.
pH and Filtration: Getting Clarity
Some products need a push into solution with acid or base. Adjust slowly, measure with a meter, and shoot for a range suggested by protocol, not a guess. Overshooting pH means starting over. I’ve learned that filtration, using a membrane filter or syringe filter, clears lingering particles. Unfiltered solutions turn up surprises during measurements, especially in sensitive assays.
Aliquoting and Storage
Splitting the final solution into smaller vials saves hassle during future experiments. Contamination and repeated freeze-thaw cycles break down even stable products, which can throw off results in unexpected ways. Clear labeling—both with date and concentration—ain’t just for neat freaks, it keeps future confusion and mistakes down. A crowded freezer or fridge screams for readable labels.
Finding the Right Path Forward
Every protocol benefits from reviewing literature and talking to peers who’ve run successful experiments before. Published methods, when followed, guard against silent errors. Some labs post step-by-step instructions on the fridge door, and I’ve lost count of how many times those printouts have set me straight after a misstep. Improvements come from feedback and troubleshooting, and one experiment’s success lays groundwork for the next.
No Shortcuts in Good Science
Cutting corners leads to ambiguous or misleading results. Extra care during each step clears the path for solid data. Respecting the product and the process builds trustworthy research. Diligence in preparation pays off long after the experiment finishes, backing up findings with confidence.