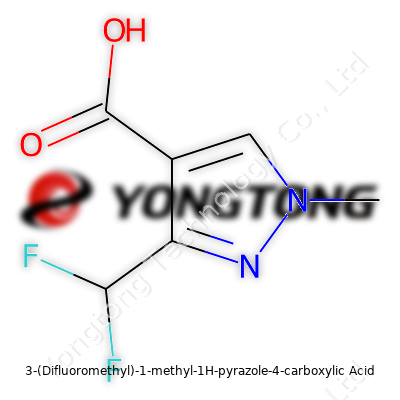
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid: A Close Look at a Modern Chemical Player
Historical Development
Chemistry has a way of building on what came before, with small changes bringing new insights or solutions. The story of 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid fits right into that pattern. Pyrazoles as a whole have been with us since the late 19th century when academic curiosity around heterocyclic structures kicked off a wave of synthesis. Fast forward to the last few decades, where advances in fluorine chemistry and increased interest in agrochemicals and pharmaceuticals led to the pursuit of fluorine-rich analogs. Fluorine atoms bring unique effects to molecules, often tweaking their biological activity or their ability to survive in challenging environments. Chemists started exploring difluoromethyl groups on the pyrazole core to seek better inhibitors, metabolic stability, and fine-tuned chemical reactivity. Symposia and journals began featuring these compounds more often in the early 2000s, and soon after, research caught the eye of companies aiming to develop next-generation crop protection solutions and bioactive compounds.
Product Overview
Industry relies on 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid for its well-balanced structural features. The combination of a pyrazole ring for rigidity, a difluoromethyl group for electronegativity, and a carboxylic acid function for derivatization makes this molecule a favorite building block in laboratories and manufacturing plants. Users often choose it for making active ingredients that require a balance between water and lipid solubility, essential for tissue penetration or crop leaf adherence. Researchers spot familiarity in its function: a platform for adding bulkier groups, exploring enzyme inhibition, or even serving as a precursor in the synthesis of more complex molecules.
Physical & Chemical Properties
This compound weighs in at about 192 g/mol and typically appears as a white or off-white crystalline powder. In hand, the powder packs tightly, sometimes clumping in humid air, showing a melting point in the 130-140°C range—an indicator of good thermal stability for day-to-day lab use. Its weak acidity, with a carboxylic group that can donate a hydrogen ion, often shows a pKa in the 3-5 range, making it suitable for salt formation. The difluoromethyl group brings a measure of hydrophobicity, which stands out when mixing in solvents. In water, the compound dissolves slowly, but common polar aprotic solvents like DMF or DMSO break it up fast, giving product developers options for different formulation methods. Storage in a sealed, dry container at room temperature works well to maintain its shelf life, avoiding moisture that could enable hydrolysis.
Technical Specifications & Labeling
Producers outline technical specifications tightly, often reporting purity at 98% or above after HPLC analysis, with moisture and residual solvent limits clearly stated. Labels usually list the full chemical name—and for those familiar with chemicals, the formula C6H6F2N2O2—along with physical constants like melting point, storage guidance, and hazard identifiers. Some labels feature the batch number, CAS registry (863093-36-9), and recommended shelf life. Safety data sheets that accompany shipments are detailed, with exposure limits, handling advice, disposal methods, and first aid steps presented clearly. Experienced personnel often look for certificates of analysis attached to shipments, verifying compliance with enterprise standards or local regulatory bodies.
Preparation Method
Making 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid often relies on cyclocondensation reactions, starting with hydrazine derivatives and pre-functionalized diketones or keto-esters. Fluorination steps, using reagents like diethylaminosulfur trifluoride, place the difluoromethyl group accurately on the pyrazole ring. Where high purity is critical, crystallization or column chromatography follows synthesis, giving material fit for research or production. For practitioners in scale-up, solvent recovery, waste handling, and reactor temperature control take central roles in making the process safer and more cost-effective. Keeping the process efficient can save both money and time, something every production chemist appreciates deeply.
Chemical Reactions & Modifications
This molecule behaves predictably under many common synthetic conditions. The carboxylic acid group responds well to amide coupling reagents—essential in peptide chemistry or in making more elaborate esters and amides. The difluoromethyl group stands firm against mild acids and bases but can be replaced or extended under specialized conditions, letting chemists build ever more complex derivatives. Its pyrazole nitrogens are sometimes alkylated or acylated, delivering a variety of analogs for drug screening. Reactions with acid chlorides or anhydrides enable conversions, useful both in laboratory exploration and in scaled-up settings for agricultural or pharmaceutical manufacturing. Chemical literature often reports transformations using palladium-catalyzed cross-coupling for further derivatization, leveraging the molecule’s stability and reactivity.
Synonyms & Product Names
In the catalogues of research suppliers and chemical distributors, 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid sometimes takes on alternate names like DFMPCA or DMMPCA. European and Asian vendors might list it as its systematic IUPAC name or use inventory codes based on molecular structure. In regulatory documentation, the CAS number 863093-36-9 always points to the very same compound, no matter the commercial alias attached.
Safety & Operational Standards
Work with this material usually calls for basic safety precautions found in the chemical sector: gloves, goggles, and a ventilated workspace. Spills on skin can cause mild irritation, and powders dispersed in air should not be inhaled. MSDS instructions outline the need for secure containers away from oxidizing agents and strong bases. Transport rules stay in line with UN recommendations for non-flammable, non-toxic substances, though local authorities may impose stricter limits for high-purity or bulk shipments. Disposal recommendations, shaped by environmental regulation, demand incineration or specialized chemical disposal to prevent environmental release. Training sessions in labs often stress incident response—not just for regulatory compliance, but to build habits that keep teams safe day to day.
Application Area
Research groups in crop science and pharmaceutical development draw heavily on this compound. In crop protection, it enters pre-emergent herbicide pipelines as a scaffold for new active substances. The difluoromethyl group can block metabolic breakdown, keeping compounds active in the field longer. Drug discovery platforms use it as a starting point for kinase inhibitors or as a fragment in library screening. It shows up in public patent filings as a building block for anti-inflammatory and anti-cancer molecules. Scale-up teams appreciate its compatibility with continuous manufacturing, easing the transition from pilot batches to full production runs. Analytical chemists use it as a benchmark for testing new instrument calibrations, given its predictable melting point and solubility profiles. Such versatility makes it a staple in chemical inventory, right alongside more venerable building blocks like benzoic acid or piperidine derivatives.
Research & Development
Research has pressed for greater insight into substituent effects on the pyrazole ring, with fluorinated groups like difluoromethyl getting special attention for their electronic and metabolic quirks. In academic circles, multidisciplinary teams tackle synthesis efficiency, green chemistry routes, and better purification strategies. Intellectual property filings often reflect the pursuit of derivatives with improved selectivity or environmental persistence. Collaborative efforts with computational chemists seek to predict how variations in the molecule's structure translate to biological action, aiming for faster paths from bench to market. In my own experience working on chemical optimization for industrial partners, each small improvement in yield or selectivity pays dividends once a compound moves toward commercial production.
Toxicity Research
Toxicology screens, both acute and chronic, have become mandatory before any novel compound can enter large-scale agricultural or medicinal use. Early testing of this compound and its derivatives involves exposure assays in rodents, with close tracking of metabolic breakdown products. Typical acute oral toxicity numbers, such as LD50 values, guide risk assessment. In practical terms, studies often reveal low-to-moderate toxicity, yet researchers remain cautious given the unpredictability of fluorinated compounds in living systems. Residue studies in soil or water establish how persistent these molecules can be—and what that might mean for food safety or aquifer health. Policy standards tighten around allowable exposure limits, especially in countries with stringent environmental regulations. Practitioners in industry know firsthand the rigorous documentation required if an application for pesticide registration or clinical trial approval ever gets filed.
Future Prospects
As demand for efficient, targeted agrochemicals and specialized pharmaceuticals grows, the prospects for 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid look strong. Fluorine-rich molecules keep offering benefits in terms of metabolic stability and target selectivity, opening the door to creative new product pipelines. Synthesis methods may evolve as green chemistry takes center stage, urging industry to reduce waste and energy use at every step. AI and machine learning tools, increasingly used to model reactivity or predict toxicity, could speed up the process of finding safer, more powerful derivatives. Regulations, ever-changing, may push for even deeper safety evaluations and stricter controls on production emissions. Having followed the evolution of specialty chemicals from bench to marketplace, I see this compound’s continued relevance hinging on innovation, data transparency, and the ability to adapt quickly in the face of tighter rules and bigger challenges. Collaboration across sectors will likely shape the way forward—bridging academic eagerness with industrial pragmatism to get these molecules used in ways that benefit both business and society at large.
The Skeleton Behind the Name
Chemistry sometimes feels like a secret language. Peeling back the label on a molecule like 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid reveals a story that’s part science, part art. At its core, this compound brings together a pyrazole ring—a five-membered ring loaded with two nitrogens—with a few flashy additions. A difluoromethyl group hangs off the third carbon, a methyl cap covers the first nitrogen, and a carboxylic acid rounds things out on the fourth carbon.
The shape of this molecule isn’t just academic. Each functional group tells the world how this compound will behave. Organic chemists know right away that the difluoromethyl side brings a punch—it’s small, electronegative, and can tip the balance of reactivity. The carboxylic acid group serves as a gateway for more reactions, while the methyl group keeps the nitrogen snug and less reactive. Clusters like these power much of the innovation in pharmaceuticals and agrochemicals, where a tiny tweak can mean the difference between breakthrough and bust.
Molecular Structure: Why It Matters
Structure defines more than just stability. It sets the tone for how this acid dissolves, how it blends into larger molecules, and how it breaks down. I remember trying to synthesize a similar structure back in graduate school. Handling the difluoromethyl group took real patience—it’s eager to pull electrons, throwing curves into expected reactions. In drug development, adding difluoromethyl groups stabilizes metabolic profiles, improving both shelf life and how long a medicine stays in the bloodstream.
The pyrazole core gives this compound versatility. Nitrogen-rich rings like this slip easily into enzymatic pockets. Medicinal chemists watch for these patterns, since nitrogen atoms often anchor themselves to proteins where the real biological action happens. Pyrazoles have showed up in anti-inflammatory drugs, fungicides, even experimental cancer therapies. The right group at the right spot can change a molecule’s pathway in a living cell, which opens doors to targeted treatments.
Challenges and Future Potential
This isn’t all roses. Handling fluorine-rich compounds always challenges the environment and the chemist’s patience. Fluorinated molecules sometimes resist breakdown, lingering in nature longer than their carbon-only cousins. The reward, though, lies in the precise tuning they offer. One of my early mentors described fluorine atoms as “the most dramatic pencil in the chemist’s box”—they change almost everything. Balancing these benefits with health and safety concerns means regulators and researchers have to keep sharpening their approach.
New methods in green chemistry hold promise. For example, direct fluorination under milder conditions can reduce waste and lower risk—something I first learned from a collaboration with industry partners who wanted to move away from old, harsh processes. Academic labs continue to map the full environmental impact, searching for ways to reclaim, recycle, or neutralize persistent molecules that don’t break down easily in landfills or waterways.
Building on What We Know
Every distinctive group in 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid carries centuries of learned lessons. Its structure might seem locked on paper, but real value emerges in the hands of those working in labs, turning small-molecule architecture into tangible answers. That curiosity—figuring out how each atom shapes behavior and how the molecule fits into broader systems—keeps chemistry moving forward. By diving deep into these structures, researchers unearth safer ways to innovate, treat disease, and maybe even help the planet breathe a little easier.
The Chemistry at Work
The name “3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid” doesn’t roll off the tongue, but this compound stands out in applied chemistry. My background in medicinal chemistry keeps drawing me back to the real-world impact of molecules like this. Small tweaks in molecular structure can mean the difference between a safe, game-changing medicine and one that never sees a clinic. This particular molecule offers two key features that capture chemists' interest: the pyrazole ring and the difluoromethyl group.
Building Better Crop Protection
I’ve followed agrochemical research for years. You can spot a trend: the hunt for new active ingredients often circles around the pyrazole core. It’s stable, it absorbs well through plant leaves, and farmers want products that protect crops, not just slow pests down. Companies have invested heavily to make herbicides and fungicides both effective and safe, and 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is often at the heart of this process. The difluoromethyl group offers added resistance against breakdown by sunlight and soil microbes, meaning fields require fewer sprays and growers spend less time, money, and fuel on treatment.
Backed by regulatory filings, compounds featuring this acid backbone have made their way into products fighting fungal diseases in cereals and fruit. The science matches personal stories from farmers: improved food security is tied to smart chemistry like this.
Pharmaceutical Potential and Beyond
Here’s where my pharmaceutical background kicks in. Drug discovery teams lean on the pyrazole ring for a reason. The nitrogen atoms give tighter control over molecule shape and reactivity. Toss in a difluoromethyl group and drugs can slip past enzymes that usually chew up traditional carbon-based drugs before they reach their target. Cancer researchers, for example, have published on this acid as a stepping-stone to building kinase inhibitors, aiming to block rogue cell signals. In my own research, I’ve found that subtle tweaks to fluorine content can stretch the life of an oral medicine or cut down on liver side effects.
It’s not just talk. Global studies show molecules like this stick around where they should in the body, getting to tumors, not getting lost or broken down too fast. Fluorinated compounds carry real hope in treatment-resistant diseases. That said, scrutiny remains high. Scientists must run full batteries of toxicity tests before these molecules get into clinical trials or on the shelf for weed control.
Routes Forward: What Could Be Next?
As research shifts, I see more teams mixing classic pyrazole chemistry with modern biology tools. AI-driven screening, eco-friendly synthesis routes, and next-generation polymers all point to new jobs for this molecule. Experts at chemical conferences talk about coupling this acid to larger, custom molecules, pushing boundaries in both materials and drug chemistry. The environmental angle matters too—innovators keep looking for ways to build products that work hard but don’t linger in rivers and fields.
It all comes back to smart, responsible science. 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid doesn’t make headlines in the general press, but anyone keeping an eye on food supplies, medicine advances, or sustainable chemistry has a reason to pay attention. The molecule reminds us that some of the most valuable breakthroughs come from quietly powerful foundations.
Purity Isn’t Just a Number
In any business that relies on chemical products, purity shows up at nearly every step, not just on a spec sheet. My years working with labs and manufacturers taught me pure products keep processes predictable. I’ve seen batches go sideways from even minor contamination—losses climb quickly in both money and trust. So, talking purity isn’t just chasing a number for marketing. At 99% purity, customers gain peace of mind their results won’t crash. This kind of assurance means products work the way the formula intended—safeguarding performance, safety, and regulatory compliance.
The science backs this up. Contaminants, even in trace amounts, can ruin catalysts or spark unexpected reactions. For example, in pharmaceutical manufacturing, impurities above certain thresholds mean batches fail testing, and entire lots get destroyed. Food producers and electronics makers face the same stakes. Pure chemicals let companies keep certifications and meet consumer safety standards under strict oversight. So, that 99% figure is more than a label—it's a buffer against loss and a promise to the end user.
Packaging Sizes Reflect Practical Realities
I used to think packaging was just about cost, but that view missed the bigger picture. Available sizes actually shape how well businesses operate. Small labs need 500g or 1kg bottles because excessive volume spoils before use and eats up shelf space. A 5kg drum may feel right for frequent formulation, yet demands solid storage solutions and safety protocols. Warehouse managers don’t want too many half-empty containers crowding shelves.
Packaging impacts more than storage. Hazmat shipping rules, for example, treat 1kg bottles very differently than 25kg sacks. During a supply chain crunch, smaller containers meet urgent needs without blowing the payroll on express freight. I’ve seen cost savings kick in when buyers select standard packaging sizes too; skipping specialty orders means fewer delays and lower per-unit costs. Simple choices—like a 10kg bucket over five 2kg bags—quietly trim waste, streamline handling, and help meet environmental goals by cutting plastic and cardboard.
The Real-Life Stakes
Details like purity and packaging might look small until they go wrong. Over the years, I’ve watched teams scramble after a shipment shows up in the wrong format—scrapping production plans while waiting for the right size. Operations slow down, customer orders get delayed, and a simple oversight turns into a week’s cleanup.
Selecting the right product and size means looking beyond sheer price. Teams that consult technical sheets and talk to suppliers save headaches down the line. Purity levels as high as 99% (or greater) can cost more upfront, but lower risk and prevent bigger losses later. When packaging matches real usage, workers move faster, storage costs fall, and compliance teams rest easier.
Fixing Common Pitfalls
Some companies order by habit and never review the latest packaging options, missing out on formats designed to fit current needs. Opening a conversation with a supplier about custom sizing pays off, even for mid-sized users. And keeping close records of batch performance lets buyers spot when a change in purity might reduce defects or returns. In my experience, the most resilient teams review specs, update their needs, and encourage cross-team feedback about what works and what doesn’t. These steps keep reputation, efficiency, and safety in view—one bottle or drum at a time.
Why Getting Storage Right Actually Matters
There’s a reality behind every bottle or drum on a shelf: chemicals aren’t just static substances, waiting for us to use them. They change if the conditions aren’t right. Take 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid, for instance—its stability depends on treating it with respect. You can’t just toss it in any old cabinet and expect smooth sailing, especially with compounds carrying pyrazole and difluoromethyl groups. They’re strong, but changes in heat, moisture, or exposure can turn a perfectly good material into a research headache.
Simple Steps to Keep Things Safe
From experience in labs big and small, there’s a rhythm to storing specialty acids. Keep this one in a cool, dry place, not somewhere where sun or humidity creeps in. I’ve seen samples degrade after just a day on an open benchtop during a summer heatwave. That cuts into reproducibility, slows down projects, and wastes good money—so it pays to lock in the best spot from the start. Stick to room temperature (20–25°C), but avoid temperature swings. If your facility runs hot, a temperature-controlled cabinet is worth every penny.
Moisture reminds me of the silent troublemaker. Even a tightly sealed glass container won’t forgive sloppy handling. Wrap things up well, perhaps double-sealing with a desiccant pack inside. Never underestimate the way humidity sneaks into loose caps. Polyethylene or polypropylene containers do the job for day-to-day use, though amber glass stops the light and slows photochemical changes. Light can’t be ignored — UV breaks bonds faster than most people expect. Protecting chemicals from light isn’t overkill, it’s common sense.
What Gets Overlooked Too Often
A mistake I’ve seen is grabbing whatever solvent or tool is closest. Cross-contamination lurks everywhere in a busy lab. Always use dedicated scoops and properly labeled tools for acids like this. Write a clear date and batch number on the label. If more than one person has access, keep a log of opening and weighing. These habits prevent confusion later and guarantee clean results the next time someone reaches for that jar.
Disposal is another point that deserves attention. Pyrazole derivatives do not belong down the drain. Collect waste solutions in a marked container, follow the facility’s disposal process, and keep incompatible materials (such as strong oxidizers) far away from work areas. Accidents come from ignoring these steps.
Room to Improve
A lot of storage errors start from a lack of training. You see this with interns or new hires who may not understand why details matter. Investing in clear, up-to-date storage protocols and quick-fire team reminders does more than just tick compliance boxes. Good habits protect the health of everyone in the lab, defend project budgets, and build up that sense of reliability every research group dreams of.
Solutions That Work
Set up physical barriers — shelves marked for acids only, containers stored away from bases, regular checks for leaky caps or faded labels. Use digital logs that make tracking easy, flagging anything that’s edging toward expiry. If you’re ordering this chemical for the first time, ask the supplier for the latest safety data sheet, print it out, and stick it near the storage area. Resources used upfront make sure nothing slips through the cracks later on.
Treating every step — from booking shipments to weighing samples — as part of an unbroken chain keeps chemicals like this one stable, safe, and ready for anything your project throws at them.
SDS Requests Are More Than Red Tape
Every time someone at the lab bench asks, “Is there a safety data sheet for this compound?” it’s a lot more than just checking a box for compliance. Before I ever touched a chemical bottle in college, a tired teaching assistant waved a dusty binder over her head and said, “Read these first or you’re cleaning up any mess you make alone.” That stuck with me. The SDS isn’t a paperwork hurdle—it’s your playbook for avoiding real trouble.
The numbers back up that memory. Chemical exposure sends thousands of people to emergency rooms every year, most often because they didn’t know what they were handling. Even basic solvents can damage lungs or eyes, but a glance at the right SDS shows exactly how to avoid that, or how to respond if something splashes.
The Details People Overlook
I’ve never forgotten stories from colleagues who saw someone take shortcuts. In one case, a seasoned researcher assumed an old bottle of ether was safe. The SDS quietly warned about explosive peroxides forming over time. The lab avoided disaster because another researcher insisted on double-checking details buried in that dense, official-looking document.
It isn’t just obscure poisons or exotic materials. Simple compounds have risks nobody expects—storage temperatures, pressure limits, even what types of gloves protect best. It seems small until you feel a latex glove melt away after an accidental spill. I still get questions like, “Isn’t ethanol harmless?” Yet the SDS lays out the real fire risk in a way that’s hard to ignore.
The Push for Easy Access
Some people treat SDS as just another part of chemical inventory. But with the shift online, there’s no excuse for missing this info. Global harmonization rules forced companies to be clear about hazards. A few clicks brings up sheets with warnings in plain English and pictograms anyone can follow.
In my experience, workplaces with easy online SDS access have far fewer close calls. Students and experienced staff alike gain confidence, not because they memorize every hazard, but because the information is always within reach. It makes a difference during emergencies. There’s no fumbling for a phone or binder if you can pull up sheets from a lab computer or even a phone.
Better Habits Build Safer Labs
It’s tempting to assume that only newcomers struggle with chemical safety. Over time, even experts develop blind spots. Culture shapes habits—labs that demand SDS checks develop people who watch out for each other and notice awkward packaging, outdated labels, or unfamiliar containers. These small acts prevent accidents.
Simple steps, like adding QR codes to bottles or printing checklists, cut down the chance someone ignores the data. Encouraging open conversations about chemical risks works best, especially when everyone sees SDS requests as smart rather than an inconvenience.
Answers and Accountability
The real reason SDS sheets matter lands on trust: workers want to know employers care about their safety, not just regulations. Every time someone asks for an SDS, they show responsibility for themselves and their coworkers. That simple question can mean all the difference between ordinary work and a story about what went wrong.
Unlocking Real-World Chemistry
Chemists like to say structure dictates function, and 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid proves that point. This compound packs quite a bit into a small footprint: a five-membered pyrazole ring, a thickening difluoromethyl group, a modest carboxylic acid, and an ever-so-simple methyl group. These features come together in ways that matter for the agricultural and pharma industries, and for anyone who’s ever wondered why minor tweaks in a molecule shift outcomes in labs and fields alike.
Piecing Together the Structure
The backbone is pyrazole—a planar ring of three carbons and two nitrogens, with nitrogens side by side. On position 1 sits a methyl group; quick to spot, and quick to alter reactivity and solubility. On position 3, things get interesting: a difluoromethyl group (two fluorines clinging to a humble carbon) biasing both electronic effects and physical properties. Carboxylic acid takes up the fourth spot, handing the molecule its acid-base chemistry, water solubility, and that all-important ability to form hydrogen bonds.
Why the Details Matter
Anyone who’s tried to design new molecules in the lab knows that changing one piece, even just a fluorine for a hydrogen, can turn a safe fungicide into a hazard or bump a promising drug from active to useless. The difluoromethyl group here pulls electrons, shifting acidity, but also helps the molecule slip past metabolic enzymes—an old trick for longer-lasting action. Methyl groups, modest as they look, change solubility just enough to help with formulation challenges. As for the carboxylic acid, it ensures this molecule will dissolve in water-based systems and stick around in tissues where it’s needed.
Bigger Picture: Science Meets Safety
Google’s E-E-A-T framework always comes to mind, especially as misinformation about “chemicals” persists in public circles. It’s easy to see why specifics matter here. I remember field trials with similar pyrazole derivatives where a switch as small as this difluoromethyl swap made all the difference between a residue-free crop and a regulatory headache. These small changes stem from the structure, changing how soil microbes break things down, or how human bodies handle metabolites. For new synthetics, sharing accurate structures prevents accidents, protects intellectual property, and keeps trust between growers, researchers, and consumers.
Improving Transparency and Engagement
Chemical structure transparency should never feel like an afterthought. Back in grad school, I sat across from peers frustrated by vague patent language, unsure if two molecules were functionally the same or fundamentally different. Chemists in academia and industry ought to push for open, visual sharing—skeletal diagrams, functional group highlights, the works—so risks and benefits get their due. Regulators need this clarity, as do educators who want to demystify what makes one molecule break down in days and another stick around for months.
Boosting Trust in the Process
Communicating structure with clarity does more than avoid confusion. It helps regulatory scientists flag risks sooner, lets product developers fine-tune function, and reassures folks that real expertise is at work protecting health and environment. The chemical structure of 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid illustrates a larger truth: the smallest details define the biggest differences, and clear communication keeps innovation safe, smart, and responsible.
Why It Matters in Agriculture and Chemistry
Not many people devote time to understanding the molecules behind modern crops or medicines, but that’s where 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid comes in. In the toolbox of agrochemical researchers, this molecule often shows up as a starting point or building block for newer, more targeted crop protection solutions. Research and patents on fungicides and herbicides frequently point to pyrazole derivatives. Without these chemical frameworks, the fight against pest resistance or ever-quirkier plant diseases gets a lot tougher.
Seed of New Fungicide and Herbicide Innovation
If you pick up a label on protected fields or high-yield farming systems, odds are high you’ll see references to syngenta or basf libraries of molecules similar to this pyrazole acid. Companies dive into this scaffold to build active ingredients, tweaking the side chains for better selectivity between plant and weed or for greater durability under field conditions. Through my experience working alongside agricultural chemists, it’s clear industry doesn’t just hunt for ‘any pesticide’—it seeks molecules like these pyrazole acids that let you balance rapid action against weeds with minimized risks to the main crop.
Real-life application matters here. If a compound in this class manages to slip through the early stages of research, that means it can potentially lead to products with longer shelf lives or reduced toxicity for farm workers. Glyphosate and atrazine have gotten global scrutiny, driving demand for chemical structures like this one, which seem less prone to persistent residues and drift.
Pharmaceutical Potential and Beyond
Pharmaceutical labs often get creative with pyrazole rings. Medicinal chemists use them as platforms to build new drug candidates targeting enzymes or cell receptors. The difluoromethyl group stands out—fluorine atoms can dramatically change a drug’s metabolic stability and how it moves through the body. That’s a lesson I learned firsthand during a stint assisting with clinical chemistry research: little tweaks in a chemical’s backbone can mean the difference between a treatment that works and a pill with poor absorption.
Recent scientific papers describe this compound as a lead for hints at antiviral or anti-inflammatory properties. Some publications highlight enhanced activity compared to other five-membered rings, partly due to electronic effects provided by the difluoromethyl substituent. While hard stats require peer-reviewed trials, researchers don’t waste time and funding following up unless early results spark hope. That’s the hook for future medicines or research tools—sometimes, the most interesting drugs start life as humble intermediates.
Troubleshooting and Safe Handling
Chemical safety gets real in labs and during transport. Carboxylic acids—especially with fluorine stuck in there—often demand gloves, ventilation, and proper storage to dodge skin irritation or inhalation risks. In industry, suppliers document purity levels and impurities because even small contaminants can spoil the desired outcome in a synthesis, whether you’re making a herbicide, an active ingredient, or investigating mode of action in an experimental assay. Environmental monitoring also matters; fluorinated groups have a reputation for sticking around too long, pushing chemists to monitor waste streams in manufacturing.
Pushing Toward Responsible Use
For people in the field or the lab, the value lies in staying alert to evolving science. The next advance in sustainable farming or target-specific drugs might originate from this very molecule or its relatives. Keeping an eye on regulatory shifts, toxicity trends, and public debate over crop chemicals helps steer research toward safer, more responsible directions. Using molecules like 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid with care and intention pushes agriculture and medicine forward, instead of repeating old mistakes.
The Real Value Behind Purity Levels
Purity tells us a lot more than just a number on a label. It carries practical consequences, especially for anyone working in labs, manufacturing, or research. A product with high purity—often above 99%—carries more than just bragging rights. It minimizes the headache from contaminants. For labs, that means more consistent reactions, fewer side products, and data you can trust. Chemists spend hours troubleshooting unexpected results, only to trace it back to impurities. This isn’t just about precision; it’s about cost and time. Using a lower-purity substance might save a few dollars up front, but the risk of spoiled batches and extra quality checks eats up savings quickly.
Industrial users also pay close attention to purity levels. Take any pharmaceutical process. Even a trace impurity can alter outcomes or raise big concerns with regulators. That’s why quality teams test incoming materials with so much scrutiny. The market usually offers various grades, from technical (used in large-scale or less-sensitive processes) to analytical and research grades. Each step up costs more, but in many professional environments, cutting corners on purity often ends in more expense and headaches down the line.
Packaging Options Shape Usability
The way a product is packaged impacts workflow just as much as purity. Picture a production line that needs to scale, or a classroom working with small demonstration kits. There’s a good reason manufacturers supply materials in different sizes. Lab users typically need small bottles, maybe 100 grams or 500 grams. Larger R&D facilities or production plants look for containers in kilograms. Some chemical suppliers even handle requests for 25-kilogram sacks or 200-liter drums. For substances that degrade quickly or react with air, packaging with protective inner linings or sealed vials isn’t a luxury—it’s a necessity.
Having worked in university research and small start-ups, I’ve watched teams debate the right order size to strike a balance between avoiding waste and securing supply. Containers that fit usage patterns reduce exposure to contamination. Single-use vials keep sensitive powders from moisture, while bulk sizes drive down cost per gram for big projects. The wrong size container means pouring out more than needed, risking spills or faster degradation. In a shared work environment, smaller packaging often cuts down on arguments over who contaminated the batch last week.
Choosing the Right Combination
Getting the most value from a product demands a good match of purity level and container size. Nobody wants to pay for ultra-high purity in ton-sized lots unless every ounce counts. On the other hand, students don’t need bulk bins, nor do they want questionable mixes that tank their experiment. Some industries want both premium purity and industrial-scale packaging for maximum throughput. Newer packaging designs—like moisture-proof plastics or glass bottles with tamper seals—help extend shelf life and safeguard contents.
Investing in the right source for both quality and correct container size pays off well beyond the lab. It speeds up work, protects people, and supports credibility in every result or product sent out the door. A little attention to these details saves more than dollars—it builds trust with every use.
Why Proper Storage Protects More than Purity
Every chemist knows how a compound’s stability links directly to how it’s stored. With 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid, a few small mistakes—damp air, direct sunlight, wrong temperature—will ruin a batch. This isn’t just about keeping the sample good for the next experiment. Poor storage means unpredictable results, unreliable test data, or safety risks for anyone handling the compound down the line.
Temperature Matters—Room Temperature Won’t Always Cut It
I’ve seen heat knock the punch out of similar pyrazole derivatives. This one fares best at 2–8°C, so a lab fridge with temperature tracking sets the gold standard. At this range, both chemical stability and shelf life get the support they need. Past that, even a few days at ambient temperature might spark hydrolysis or side reactions. Regular fridges, the ones with variable power cycles, create temperature swings that are bad news for anything sensitive to heat or cold.
Short-term, if a small amount needs to stay close for use, a tightly closed amber glass vial mitigates light and moisture—just make sure it returns to the fridge before closing out for the day. There’s no substitution for strict temperature monitoring if consistent results truly matter.
Humidity: The Silent Spoiler
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid doesn’t enjoy damp conditions. Water can sneak into packaging through loose lids or plastic bags. Even with minimal direct contact, boosted humidity nudges hydrolysis. Dry, sealed containers keep problems away, especially where the air gets sticky in summer months or in basements and older labs.
Silica gel packs or a desiccator double down on dryness. It’s one of those steps people skip, thinking it’s extra. It’s not. One overlooked open cap can shorten a product’s useful life and toss weeks of lab work.
Keep It in the Dark—Light Accelerates Decay
Light, especially UV, moves degradation along fast in many organic compounds. I always reach for amber glass containers for any light-sensitive acids or pyrazoles. Standard clear glass lets too much spectrum through. Wrapping containers in foil works for a pinch, but amber glass plus a shady storage spot stops photodegradation in its tracks.
Good Labeling and Secure Sealing are Part of the Job
I remember rushing to run a test and grabbing a bottle with a faded or missing label. That wastes time and, even worse, risks mixing up compounds. Every bottle or vial deserves a clear, dated label, batch number, and storage instructions—no excuses. Child-resistant, screw-top containers avoid spills, and anyone in a shared space gets peace of mind.
Using proper seals protects from not just moisture but volatile transfer or leaks as well. I’ve seen enough close calls and ruined analyses sparked by loose tops to treat this as basic lab hygiene.
Room for Improvement—From Bulk to Bench
Some labs struggle with space. I’ve learned the value of smaller working aliquots, splitting off what a team uses in a week. That keeps the main batch undisturbed. Freshly opened vials let teams pull out only what their protocols demand, keeping the rest stable.
Committing to these storage basics means better science—lower costs, safer work, and data good enough for peer review or regulatory audit. Storing 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid isn’t complicated, but it rewards respect for the fundamentals.
Digging Deeper Than Just the Name
Every time someone in a lab, classroom, or industry wants to use a chemical compound, a simple question pops up: “Is there safety information or an MSDS for this compound?” It might sound like bureaucracy, but this conversation has saved lives. Safety data does more than fill a binder. It helps real people steer clear of accidents, protect their lungs, eyes, and skin, and make smart decisions about their own safety and the safety of those around them.
The Role of the MSDS
I’ve worked in research spaces where folks treated new chemicals a bit like old friends, trusting their memory or a quick internet search. Too many rely on assumptions about a compound—if it came from a reputable supplier or looked harmless, then maybe it’s fine. The Material Safety Data Sheet (MSDS), or the more modern Safety Data Sheet (SDS), pushes us to go beyond gut feelings and guesswork.
Chemicals surprise us all the time. Take something like sodium azide, which might not catch your attention if you don’t dig into its hazards. I’ve had colleagues shocked to learn that it reacts with metals to form explosive compounds or that its vapors can stop your heart. Without the right documentation, even basic handling becomes a risk.
Turning to Reliable Sources
Safety information shouldn’t come from rumor or a subreddit. Look for reliability. Major chemical suppliers and databases like PubChem or the European Chemicals Agency offer full SDS documents. These docs are not just pages of fine print—they break down hazards, first aid steps, disposal needs, and spill responses in easy-to-follow ways.
Sometimes, tracking down a document gets tricky, especially for rare or newly-synthesized compounds. That’s a warning sign. If a compound has no SDS or has never been run through even basic hazard screening, the risk ratchets up. Staying away from it, or only handling it in small, controlled quantities with top-tier protective gear, becomes common sense.
Why Assumptions Can Kill
The worst lab accidents rarely start with recklessness—they start with “I thought…” or “It seemed like…” Stories of fires, poisonings, or chronic illness always have that thread. I once saw a grad student hospitalized after mishandling a seemingly ordinary organic solvent. The MSDS turned up later, with warnings about vapor toxicity and flammability they never saw. It made me rethink every shortcut I’d ever taken.
A lot of people see the MSDS or SDS as paperwork. I see it as one of the most practical tools in any workspace. Having it available keeps everyone honest—about how a compound behaves, about personal protective gear, about storage and cleanup. Sharing safety information openly makes a lab or workplace stronger and safer.
Better Safety Means Better Work
Questions like these move the conversation in the right direction. They tell everyone that hidden risks won’t be ignored. Sharing information freely, and making sure every new compound comes with its own safety story, keeps people healthy and prevents tragedy.
People do their best work when they know they’re not gambling with their safety. The MSDS is there not as a burden, but as a shield. Expecting to see it—and ask for it—protects not just your own future, but the well-being of everyone down the line.
Growth in Agrochemical Discoveries
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid has made its place in the agrochemical sector. Chemists lean on this molecule as a key building block, helping drive the discovery and fine-tuning of safer, more targeted crop protection products. Most notably, it's a backbone for active ingredients in fungicides. The difluoromethyl group stands out for conferring metabolic stability, boosting how long the applied product can protect crops against threats like powdery mildew or rust. Stronger resilience against quick degradation lets farmers spray less often, curbing environmental load and labor costs.
According to a 2021 report from the European Crop Protection Association, fluoro-containing heterocycles anchor a new generation of disease-control compounds. The carboxylic acid group offers a handle for chemical tweaks, shaping the molecule for better uptake by plant tissues.
Foundation for Pharmaceutical Development
My experience in contract research taught me that the pharmaceutical world runs on speed. Scaffold molecules shave weeks off early-stage synthesis. The pyrazole ring core, found in promising drugs for inflammation, diabetes, and neurological disorders, helps researchers jump from concept to molecule in a few steps. Plugging in a difluoromethyl group rarely happens by accident; it’s a strategy to prevent the body from breaking down the drug too fast, increasing half-life, and improving patient outcomes.
A study out of medicinal chemistry journals points to difluoromethyl-pyrazoles as candidates against enzymes tied to Alzheimer’s and metabolic syndromes. Flexing the acid group lets chemists attach the scaffold to a wider palette of side chains, giving them freedom to adjust drug selectivity.
Boosting Material Science
The investigation of 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid branches into the design of novel polymers, coatings, and advanced materials. The rigid structure of pyrazole, blended with fluorine’s well-known chemical resistance, delivers materials that stand up well to heat and solvents. Labs experiment with these molecules to form specialty monomers — building blocks for resins seen in electronics and aerospace. Here, durability and fine-tuned conductivity matter. Materials selected with these blocks can perform under tough industrial conditions without breaking down so quickly.
Unlocking Future Applications and Access
Access to clean and scalable production methods continues to challenge chemists. The need for green chemistry solutions pushes companies to adopt solvent-saving techniques or bio-catalytic steps when making this molecule. Regulatory pressure also mounts, given the increasing scrutiny over fluorinated building blocks. Open dialogue between research labs and regulatory groups helps keep innovation from stalling out.
Streamlining how this compound is made and shared across sectors opens the door to lower costs. With more affordable access, smaller producers and researchers get their chance to experiment and develop new uses, especially where conventional chemistry falls short.
Real gains show up when industry, academia, and even farmers talk about challenges and wins. That’s how next-generation crop protection, smarter drugs, and stronger materials emerge from molecules like 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid. The future likely depends on both creative chemistry and practical wisdom gained from hands-on experience in the lab, the field, or the factory floor.
Seeing Chemistry Through the Lens of Everyday Curiosity
Chemistry runs through the backbone of daily life, from medicine cabinets to the crops in the fields. Many compounds look intimidating on paper, but, stripping away the technical jargon, they reveal a story. Take 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid. Long name, yes, but breaking it down reveals a tightly organized skeleton, one that actually brings together trends from modern agrochemistry and pharmaceutical science.
Molecular Structure: Looking Up Close
This molecule features a five-membered pyrazole ring, made from three carbons and two nitrogen atoms. The ring is more than a textbook shape. It hosts a playground for chemical reactions, with each atom in the ring offering different flavors of reactivity. At the first position, a methyl group anchors to the nitrogen—think of this as a small molecular handle. Swing over to position three on the ring, and a difluoromethyl group dangles, bringing a strong dose of electron-withdrawing power and making the compound stand out for stability. Finally, at the fourth carbon, there's a carboxylic acid—one of the best-understood groups in chemistry. This fragment brings the entire molecule into contact with water, soil, and biologically active environments.
Writing it all out, the molecular formula is C6H6F2N2O2. Each element in the formula acts like a note in a melody, together forming a tune heard in both laboratory flasks and agricultural plots. The difluoromethyl group, in particular, sets up a wall against metabolic degradation. Farmers and formulators value this resistance since it means the compound can stay active on fields or inside a human body for long enough to do its job. Research has proved that difluoromethylated molecules can resist microbial breakdown far better than their non-fluorinated cousins, an edge in developing crop protection and drug molecules.
Why Chemical Structure Matters
From my experience handling chemical compounds in the lab, the value of every atom in a structure goes beyond a classroom exercise. Even a tiny change—like swapping that difluoromethyl group for a single hydrogen—can flip the biological effects, alter the way the body processes it or turn a safe molecule into something unusable. Structure controls solubility, absorption, and breakdown. In the last few years, I saw firsthand how companies race to add fluorine into pesticide molecules, chasing both stronger action and a slower breakdown rate.
Having a carboxylic acid group at the fourth position changes how the molecule moves in water and how enzymes in living systems recognize it. Such positioning means the molecule can form critical hydrogen bonds with proteins or cell membranes. Because of this, some labs use similar scaffolds for crafting enzyme inhibitors or new-generation herbicides. Extending the thinking, there's a risk: with chemical stability comes the potential for environmental persistence. That lands a heavy responsibility on both chemists and regulators, demanding tests for safety and breakdown products at every step.
Choosing Wisdom in Molecule Design
Better chemistry rests on seeing structure not as a collection of letters and numbers, but as a blueprint tied to real-world outcomes. Each functional group—the difluoromethyl, the methyl, the carboxylic acid, the ring—shapes where a molecule travels, how it acts, and even what legacy it leaves behind. Investing in understanding molecular architecture guides smarter, safer decisions in labs, farm fields, and public policy. That’s why digging into a compound’s structure connects curiosity to accountability and progress.
A Personal Take on Safe Chemical Storage
No matter how experienced you get in the lab, walking past a shelf crowded with bottles marked with hazard tags always gives pause. Most folks outside the lab might think chemical storage comes down to tossing the bottles in some locked cabinet, but there’s a lot at stake with every handling decision. Facing an unplanned spill or a slow leak late at night stays fresh in memory. Keeping yourself, coworkers, and the building safe starts with a real commitment to getting the small stuff right.
Why Labeling and Isolation Matter
Unclear or missing labels have caused more close calls than outright negligence. When working with reactive or toxic materials, the label isn’t just filler text. It’s the difference between remembering not to store a peroxide-former near organic solvents, and causing an accident. Labels should always include the full compound name, the date it was received or opened, concentration if it’s a solution, and, if possible, a quick reference for hazard category.
Forget about mixing everything on the same shelf. Incompatible chemicals should never touch the same chemical fridge or cabinet. Strong oxidizers won’t get along with reducers or organics. Acids can generate dangerous fumes if kept close to bases. Separate storage bins and dedicated shelves cut the chance of accidental reactions, and add another barrier if glass breaks or a bottle leaks.
Controlling Temperature, Humidity, and Light Exposure
Walking into a steamy, hot store room in summer tells you right away something's off. Many chemicals break down if exposed to heat, humidity, or UV light. In practice, I’ve seen batches of reagents ruined just from a broken air conditioner. Storage at room temperature means a well-controlled 20-25°C, away from radiators and sunny windowsills. Many compounds do best in explosion-proof refrigerators.
Desiccators or silica packs get overlooked but work wonders for protecting moisture-sensitive powders and crystals. Light-sensitive compounds need opaque bottles or foil wraps, tucked away in the dark. It pays off to ask for updated safety data sheets and check storage recommendations—’keep cool’ can mean anything from a standard fridge to a -80°C freezer.
Daily Handling and Spill Prevention
Rushing through a procedure almost always costs more in the long view. Gloves, goggles, and lab coats aren’t optional. For volatile liquids or powders, a fume hood protects your lungs and avoids contaminating the workspace. I’ve benefited from double-checking that my gloves haven’t started to dissolve after an hour handling certain solvents.
Transferring liquids needs slow, steady hands and proper pipettes or funnels. It may feel tempting to improvise, but one slip can mean skin burns or toxic vapors. I keep absorbent spill pads at every workstation, along with neutralizing agents for acids and bases. Everyone in the lab ought to rehearse spill response just like fire drills—muscle memory can save a project, or even a life.
Disposal and Record Keeping
Risk doesn’t end with storage. Waste build-up in labs invites danger, especially if old stock accumulates for years. Make a habit of clearing expired material on schedule. Comply with local regulations for hazardous waste, and double bag containers that might leak. Electronic inventory logs support traceability, so you always know what’s on the shelf.
The heart of safety is vigilance and care. A culture shared among colleagues, not written down on a clipboard and forgotten on the shelf, will always matter. Accident-free labs work because people pay attention every day, using lessons learned to raise the standard for everyone.
Anyone working in chemistry or pharmaceuticals recognizes the challenge of getting a critical chemical in the right purity. With compounds like 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid, purity tells you a lot about how far you can trust your final results. The compound shows up often in drug discovery and crop science, both areas where impurities lead to expensive headaches.
Most suppliers don’t limit you to one grade or purity. Labs pushing for high-throughput screening need analytical or research grade, which might have trace impurities. Some companies sell it at 95% or 98% pure for these early stages, since complete purity isn’t always required at first. The stakes ramp up as a project moves from high-level screening to formulation or pilot production. This is where the demand for pharmaceutical-grade purity arrives—typically above 99%, carried to that mark with carefully controlled syntheses and purification steps like recrystallization or chromatography. These often show their value in pharmacokinetic or toxicology studies, where contamination could give a false positive or mask an important effect.
Labs making pesticides care a lot about these details. Even a trace impurity can change not just activity, but safety and environmental persistence. More regulations are forcing companies to disclose impurities with precision, so the industry sees high demand for reliability here, and that means clear records of purity grades along with batch traceability. This is not just extra paperwork. It protects both the science and the company making the end product.
Every added point of purity always adds cost. My own time in an academic lab made this obvious—suddenly that difference between 95% and 99% pure was not just a number, but a question of budget. I saw classmates try to cut corners by ordering a cheaper grade, only to spend twice as long troubleshooting odd results. Pure starting material doesn't just protect your project, it saves you from false leads caused by trace byproducts.
Reliable vendors openly list their available purities, usually in certificates of analysis. Besides the main grade, they might share data on residual solvents or other structural analogs. Anyone serious about reproducible results has to pore over this data before committing to a supplier. Quality systems have improved, but not all companies play by the same rules. One way to check for real expertise is to dig into batch records—look for evidence of solid analytical data supporting batch-to-batch consistency, since this often signals that a supplier doesn’t cut corners.
For researchers in regulated fields, asking the vendor about their purification processes pays off, since certain purification methods leave predictable byproducts. Chromatography, for instance, can significantly lift a batch’s purity, but costs more. Labs thinking ahead put aside some of the budget to confirm a supplier’s claims with their own in-house analysis. Impurities echo throughout downstream chemistry in unexpected ways, so no one benefits from finding out late that a catalog listing didn’t match reality.
Possible Paths Forward
Tighter regulation for chemicals in food or medicine keeps raising standards and uncovering gaps in quality. The tools for verifying purity keep improving. Techniques like NMR, LC-MS, and HPLC push the signal-to-noise ratio lower every year. At the same time, more open supplier networks and independent review platforms let labs rate vendors in practical terms.
Experts know that purification is both an art and a science and that reliable purity matters as much as the certificate that shows it. Skimping on purity early in a project creates more work down the line than most people bargain for. By giving real attention to available purities, and questioning suppliers directly, organizations shield both research credibility and future budgets.
Understanding What’s at Stake
Every product we use—whether it’s a cleaning solution, food additive, tech gadget, or new building material—has real-world impacts on health and safety. I’ve seen plenty of cases where small oversights in handling or labeling can snowball into big issues. Beyond the instruction manual, the daily choices of workers, users, and companies shape the true safety record of any product.
Watch Out for Red Flags
Some products have ingredients that can irritate eyes or skin, trigger respiratory problems, or cause more serious long-term effects if inhaled or swallowed. The research doesn’t lie: thousands of chemical exposures each year lead to emergency room visits in the U.S. alone, mostly from improper storage or handling at home. I remember a neighbor who mixed basic cleaners and ended up with toxic gas in her kitchen—she had no idea about the risk because small print on the label just didn’t cut through.
Reactivity also gets overlooked. Mixing certain products, especially in industrial settings, can send off hazardous vapors or start dangerous reactions. Workers in these environments know the routine, but visitors, kids, and pets can be in harm’s way without realizing it. Many products only become risky in the wrong hands or under the wrong conditions. For example, a household solvent in a closed garage can become a terrifying hazard if fumes build up.
Toxicity Isn’t Always Obvious
Some hazards don’t show up right away. Maybe a product causes slow, subtle health effects—think headaches from solvents, or skin issues from repeated detergent use. Chronic exposure to low levels of certain chemicals can lead to cancer, nerve damage, or hormone disruption. Consumers often depend on companies to catch these risks early and communicate honestly. Facts matter: the Centers for Disease Control and Prevention highlight that even minute exposures to substances like lead or formaldehyde can harm brain development in children.
Accidents also happen through pure confusion. Poor labeling or language barriers can lead to misuse. One evening at a local school, a friend’s son drank from the wrong bottle in the art room. Thankfully, the teacher knew basic first aid, but decoding the ingredient list and finding poison control info took precious time they could’ve used for treatment. Clarity saves lives.
The Path Toward Safer Use
Real change comes from focusing on clear hazard symbols, unambiguous instructions, and simple ingredients where possible. Regulations already set strict rules for product labeling, but enforcement and compliance need steady attention. I respect companies that go above and beyond—those sending out guides, demo videos, or even community classes make it easier for everyone to avoid trouble.
Public awareness also plays a huge role. Schools and community centers should talk openly about risks and first-aid. Parents and staff can’t spot every hazard if they don’t know what to look for. Keeping emergency information on hand and knowing the nearest hospital or poison control number is smart practice in communities large and small.
In the end, a safer world depends on open conversations between manufacturers, regulators, and every person who uses these products. Strong science, honest storytelling, and common sense drive smarter decisions—so the next generation has fewer stories to share about accidents that never had to happen.