3,5-Difluorophenylboronic Acid: A Closer Look
Historical Development
The march of organic synthesis took a strong turn in the 1950s with the recognition of organoboron compounds as reliable building blocks. 3,5-Difluorophenylboronic acid emerged on research benches as chemists started to see boronic acids delivering dependable routes to cross-coupling reactions. Laboratories gave it more attention once the Suzuki–Miyaura reaction went mainstream after Akira Suzuki’s Nobel-winning work. In those early years, scientists handled phenylboronic acid with care and patience, gradually exploring subtler variants like difluorinated derivatives. These fluorinated compounds didn’t just come out of a chemist’s curiosity but rose from the pursuit of pharmaceutical breakthroughs, where subtle electronic modifications could mean stronger drugs or smarter materials.
Product Overview
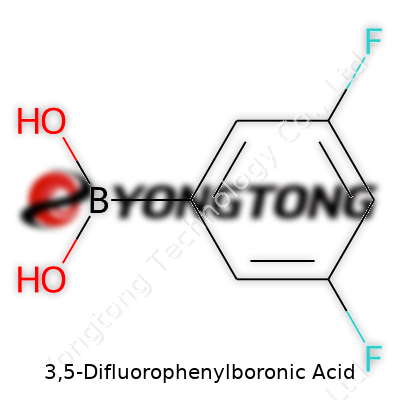
3,5-Difluorophenylboronic acid belongs to a family of boronic acids marked by their ability to build and modify molecules through organic synthesis. Its chemical fingerprint – a benzene ring with boronic acid at one position and fluorine atoms at the 3 and 5 spots – gives it special value. From small-scale labs to industrial settings, this compound sees steady demand, particularly in pharmaceutical research and materials science. Suppliers typically offer it in various levels of purity, with white to off-white powder as the most common presentation. Chemists care about this acid for its stability under ambient conditions, which means everyday handling lacks the stress that comes with some volatile precursors.
Physical & Chemical Properties
Structurally, 3,5-difluorophenylboronic acid holds C6H5BF2O2 as its chemical formula, with a molar mass sitting at around 173 grams per mole. Its melting point hovers close to 170°C, which provides a workable window in most synthetic settings. People handling it don’t worry about sudden evaporation; this powder keeps its structure until heated beyond sensible working temperatures. Water solubility remains moderate, but it prefers organic solvents like ethanol and DMSO, making it popular with those who do solution-phase synthesis. The fluorine atoms dial down electron density on the ring, subtly changing how the boronic acid group interacts in coupling reactions. Such effects make this compound predictable in the right hands, with reactivity profiles distinct from those seen in plain phenylboronic acid.
Technical Specifications & Labeling
Bottles of 3,5-difluorophenylboronic acid come marked with assay values and impurity profiles that seasoned chemists look for immediately. Purity often exceeds 97%, achieved by careful crystallization and chromatographic methods. Labels display CAS #162607-18-1, batch number, and manufacturing date. Storage instructions typically ask for a dry, cool place, away from incompatible substances like strong oxidizers. Any reputable supplier adds shelf-life guidance and safety warnings concerning dust inhalation and skin contact. Analytical data on accompanying certificates include analytical HPLC or NMR spectra, helping users confirm compound identity before critical experiments.
Preparation Method
Producing 3,5-difluorophenylboronic acid starts with a difluorinated benzene derivative, often sourced from halogen exchange on m-difluorobenzene or carefully chosen starting materials. Chemists attach boron via metal–halogen exchange—often using n-butyllithium—followed by reaction with trialkyl borates or boronic esters. Some routes make use of palladium-catalyzed borylation for direct C–B bond formation, saving steps and increasing overall yields. Crude product undergoes acidification and purification, usually by recrystallization, to reach the high purity thresholds demanded by fine chemical or pharmaceutical R&D.
Chemical Reactions & Modifications
3,5-Difluorophenylboronic acid joins organic syntheses mostly in Suzuki–Miyaura couplings, enabling the formation of carbon–carbon bonds in the construction of biphenyl motifs and heterocyclic scaffolds. These transformations have powered the production of numerous agrochemical and pharmaceutical leads. The two fluorine atoms give the core unique characteristics—smarter selectivity or greater metabolic stability in biologically active candidates. Chemists also tinker with its boronic acid group, protecting it as esters or converting it to other boron-based derivatives. In polymer chemistry, modifications of the aromatic ring can provide new functionalities for smart materials, such as sensors or catalysts.
Synonyms & Product Names
Researchers and cataloguers recognize it under several labels. "3,5-Difluorobenzeneboronic acid" comes up in some catalogs. Others list it by systematic names like "3,5-difluoro-1-benzeneboronic acid" or "m-difluorophenylboronic acid." International vendors introduce it under trade numbers or manufacturer codes, but the CAS number helps clear up any confusion when ordering or cross-referencing studies.
Safety & Operational Standards
Handling this acid demands gloves, goggles, and access to a fume hood. Even at modest scale, powder drift or accidental spills can irritate skin or respiratory tracts. The compound is not shock-sensitive or acutely hazardous, though people treat all boronic acids with respect given their organoboron core. Safety data sheets lay out emergency procedures: flush with water after skin or eye exposure, and seek medical advice if large amounts are inhaled or ingested. Facilities store it in tightly sealed bottles, away from substances that could reduce or oxidize boron-containing groups, to maintain product quality. Waste management follows local guidelines for boron-containing organic waste.
Application Area
Applications span medicinal chemistry, where the compound fuels the hunt for new drugs by linking aromatic fragments and stabilizing bright new pharmacophores. Agrochemical researchers use it in syntheses aimed at bug-resistant or drought-tolerant crops, combining the stability offered by fluorine atoms with the versatility of boronic acid coupling. In material sciences, the ability to introduce specific substitutions on aromatic rings opens doors for advanced polymers, conducting films, and even OLED technologies. Diagnostics and biosensors also tap into its availability for selective binding and detection.
Research & Development
Research sees 3,5-difluorophenylboronic acid as a springboard for further innovation. Pharmaceutical companies invest in making libraries using it as a modular core, searching for molecules that bind proteins or alter disease pathways with higher selectivity. Academics explore new cross-coupling methods or use the compound to probe reactivity in complex environments. Computational chemists model its interactions, examining how the ring’s electronic landscape changes with each substitution. Industry pushes for greener, more economical production routes, seeking to lower catalyst loading and minimize solvent waste in scale-up.
Toxicity Research
Toxicologists have explored the safety of boronic acids, noting that most display low acute oral toxicity. Still, fluorinated aromatics prompt caution, as fluorine-containing byproducts sometimes linger in biological systems. So far in rodent studies, 3,5-difluorophenylboronic acid doesn’t set off acute toxic alarms, but regulatory authorities keep a close eye on long-term exposure and environmental fate. Lab staff rely on chronic exposure data and animal studies to calibrate safe handling, ventilation standards, and waste disposal habits. Few incidents have made headlines, but broader research continues as demand for fluorinated compounds rises across science and manufacturing.
Future Prospects
Looking ahead, the demand for specialized boronic acid intermediates won’t be slowing down. Drug discovery leans heavily on variants like 3,5-difluorophenylboronic acid to tackle stubborn biological targets, especially as fluorine earns its reputation for improving drug profiles. Sustainable chemistry trends push researchers to trim the environmental cost of its production, from greener reagents to recyclable catalysts. In tech, its unique electronic and steric features promise advances in organic electronics, molecular sensing, and next-generation polymers. Lifelong learners in chemistry see 3,5-difluorophenylboronic acid as proof that even small tweaks—a pair of fluorines on a benzene ring—can turn a routine building block into an engine for creative, real-world problem solving.
The Building Blocks of Big Ideas
Chemistry shapes much of what we use and rely on, from medicine to electronics. Not every compound makes the headlines, but behind the scenes, certain chemicals hold quiet importance. One example is 3,5-Difluorophenylboronic acid. With its two fluorine atoms sitting on a benzene ring and a boronic acid group attached, the structure packs a punch for labs focused on progress.
What Makes 3,5-Difluorophenylboronic Acid Valuable
Chemists keep coming back to this compound because it boosts efficiency in key reactions. The Suzuki-Miyaura cross-coupling reaction, known for knitting together organic building blocks, runs smoother with boronic acids. Having two fluorines at those spots on the ring gives the molecule unique reactivity. In practice, this means a greater variety of carbon bonds fall within reach, letting scientists stitch together more complicated molecules. Work in my own lab taught me that choosing the right boronic acid can mean the difference between a failed reaction and a shelf full of pure product.
Supporting Drug Development
Drug companies chase molecules that can precisely interact with the body’s targets. The 3,5-difluorophenyl group brings two helpful features—fluorine atoms tweak the molecule’s electronic profile and often make the final drug more stable. Medicines containing this substructure tend to break down less easily, which makes dosing more predictable. That stability also protects against unwanted side effects from breakdown products. Many modern cancer drugs and pharmaceuticals for mental health started with research using this very boronic acid or close relatives.
Organic Electronics and Material Science
It’s not only human health on the table. 3,5-Difluorophenylboronic acid takes a seat in organic material science, giving rise to polymers and crystals with specific electrical properties. Organic light-emitting diodes (OLEDs) and solar panels built from custom-designed organic molecules require precise arrangements of atoms. The presence of both boronic acid and fluorines helps researchers lock in desired electronic behavior. After years spent fixing failed batches of materials, I learned that reliability in base chemicals translates into reliability in finished products.
SAFETY DEMANDS ATTENTION
Handling boronic acids deserves respect. Lab safety guidelines require gloves and goggles, and good ventilation isn’t up for debate. Nobody enjoys scary moments from chemical spills. Clear training on proper waste management also cuts risk and protects the environment from rogue fluoride compounds.
Challenges and Improvements Ahead
Scaling up any lab chemical, especially those vital to medicine or technology, doesn’t always run smoothly. Purity standards keep getting stricter. Supply chain hiccups, especially after global events, push research teams to consider more local or sustainable sourcing. Cleaner production methods could cut down on waste and improve access. Some chemical firms have started working on green chemistry approaches for boronic acid synthesis—minimizing byproducts and switching to safer solvents where possible. Solutions like these take teamwork between researchers, suppliers, and regulators.
The Bottom Line
Chemists who reach for 3,5-Difluorophenylboronic acid don’t do so out of habit. Its versatility and reliability support work in modern medicine and new technology alike. With continued focus on safety and sustainability, this modest molecule will keep opening doors to better treatments and smarter materials.
Looking Beyond the Numbers
Talking about chemicals, some folks get lost in formulas, pixels of letters and numbers strung together. 3,5-Difluorophenylboronic acid doesn’t roll off the tongue, but it holds steady ground in both bench research and modern drug development. The molecular weight of this small molecule—about 173.95 grams per mole—matters in more ways than folks outside a lab might guess.
Why 173.95 Really Means Something
Weighing out chemicals with real accuracy means feeling safe about every downstream step. Even a tiny math error in a synthesis batch can set off a cascade of trouble. Chemists grow up knowing the periodic table by heart, because those weights drive every calculation from dose-finding to solvent use. The 173.95 grams per mole for 3,5-difluorophenylboronic acid comes from adding up the atoms: six carbons, four hydrogens, two fluorines, one boron, and two oxygens. Nobody wants to trust a supplier or a protocol unless that number checks out.
My time in a student lab hammered this home. We’d crunch these weights, writing them into logbooks in the days before calculators lived in every pocket. Guesswork about a reagent’s purity or a decimal point shifted by mistake would cost real money, eviscerate time, and put the work at risk. Small errors multiply fast in discovery and scale-up.
Impact on Synthesis and Innovation
Boron-containing building blocks, like 3,5-difluorophenylboronic acid, shape pharmaceutical pipelines from oncology drugs to agrochemical tools. Shaving or adding just a few atoms changes the game—solubility, how tissues react, even patent claims. And all of these traits hang on top of simple arithmetic rooted in molecular weight.
For new tech, think about Suzuki coupling. That reaction, used by chemists all over the globe, lets scientists snap together custom molecules. Precise control means knowing exactly how much to weigh, how hard to push the reaction, and when to stop. Drug hunters use these weights to figure out dosing, shelf life, and scaling up without surprises. Miss the mark, and the experimental work wastes effort, forcing teams to circle back and start over.
Dealing with Purity and Verification
Traceability didn’t always get the respect it gets today. Now, digital records, purity certificates, and cross-checks are part of how pharma and academics avoid contamination and comply with regulators. Every batch of 3,5-difluorophenylboronic acid needs quality assurance to keep the reported weight honest. Labs invest in high-resolution mass spectrometers just to verify these facts, since a few rogue grams can mean the difference between a safe batch and a failed audit.
Solutions: Bridging Data and Practice
One way to lower risk is adopting cloud-based inventory systems that tag every raw chemical with its analytical documentation. Barcode tracking saves confusion when research moves fast or when lab members rotate out. Smart scales and integrated software now flag out-of-range weights and automate mole-to-gram calculations, sidestepping manual misfires. These tools keep the whole research process sharp and accountable.
Anyone handling chemicals like 3,5-difluorophenylboronic acid leans on that simple value—173.95 g/mol—as a cornerstone. It represents the hard-won trust scientists and engineers build with data, each day, molecule by molecule.
Looking Beyond the Label
A shelf full of bottles in a chemistry lab might look organized, but anyone who's worked with chemicals knows storage mistakes create real headaches. With 3,5-Difluorophenylboronic Acid, this lesson hits home. The bottle usually arrives fresh, white, and labeled, but the challenges start as soon as it enters a humid or warm space. Through my years working in academic labs, hearing about someone’s favorite organoboron sample turning brown in the corner wasn’t rare. If it gets lumpy, dark, or sticky, you’ve lost more than just money. Accuracy slips, reactions slow down, and hours vanish as you troubleshoot a reaction that just won’t cooperate.
What Fluorine Means for Storage
Compounds with boronic acids often crave a dry atmosphere. Toss fluorines onto the ring, and now moisture or oxygen can nudge them into breakdown. Reports across industry and academic sources—like Sigma-Aldrich and peer-reviewed chemistry papers—call this out. Once, a researcher next to me kept their bottle on a steel shelf near a sink. Didn’t take long for a dark crust to appear. Their yields plummeted. It isn’t just about looks—degradation throws off molecular weight and impacts catalytic cycles or Suzuki couplings. Even the best-run reaction can fall apart if the starting reagent is compromised.
Controlling the Elements
Most chemical supply companies print explicit directions for storage: “Keep container tightly closed in a dry and cool place.” That sounds simple, but busy routines and tight spaces push people into bad habits. In one startup, humidity would creep past 60% in the hot months, and “cool” turned into “room temperature…sometimes,” which means you end up paying twice: once for the wasted chemical, and again for repeating your synthesis.
Personal experience lines up with the evidence: sealed containers, stored away from windows or heat sources, protect your investment. Silica gel packs inside the storage cabinet help absorb stray moisture. In most chemistry departments, people now favor refrigeration—between 2 to 8°C gives stability. Don’t crowd the fridge with food or drinks; cross-contamination and repeated opening invite water vapors.
The Real Cost of Careless Storage
Replacing 3,5-Difluorophenylboronic Acid isn’t just about ordering a new bottle. Budgets feel the squeeze, especially at universities and small biotech outfits. The environmental impact builds up too, since disposal of a degraded chemical needs more resources and creates unnecessary waste streams. The issue runs deeper in places without strict chemical safety rules, where fumes and residues put people and the environment at risk.
Practical Solutions That Add Up
A few changes solve most issues. Ingredient logbooks help track expiration dates and label open dates. Including clear instructions near the storage area keeps newer team members from guessing. Investing in a low-cost electronic hygrometer screens for hidden spikes in humidity. Shared calendars can remind everyone when to check on storage conditions.
In the end, careful storage never feels exciting—yet every seasoned chemist recognizes its value. Save time, money, and health by respecting the quirks of molecules like 3,5-Difluorophenylboronic Acid. If you treat it with care, your results stay sharp and you dodge problems that too many people learn about the hard way.
Why Purity Tells a Bigger Story
Before trusting a batch of 3,5-Difluorophenylboronic Acid, researchers like me pay sharp attention to purity levels. I’ve seen what even a minuscule contaminant can do inside a reaction flask—yield drops, time wasted, and reliable conclusions get lost. In organic synthesis, that extra half percent of impurity sometimes means a failed catalyst or unusable intermediate. Anyone who invests weeks into a project only to get strange results learns to check material specs the hard way.
What the Numbers Mean for Researchers
Most lab catalogs list this compound at 97%, 98%, or even higher purity, measured by HPLC or NMR techniques. For many small-molecule preparations, 97% might work out fine, but I remember using a 95% batch for Suzuki-Miyaura cross-coupling and getting strange byproducts. It turned out those extra unidentified peaks in the NMR—often byproducts from manufacturing or decomposition—could have poisoned my catalyst. Purity isn’t a bragging point; it’s a necessity when you chase reproducible results.
Academic protocols usually call out the purity used, while big pharma’s standard operating procedures mandate documentation and verification. If you’re lucky, you can snag a certificate of analysis showing exactly how the manufacturer arrived at their purity figure. Transparency builds trust, and with the stakes so high where grant money and patents ride on data, nobody wants guesswork.
Less Obvious Impacts of Lower Purity
Impurities go beyond ruining a reaction. Analytical chemists constantly chase tiny differences in chromatography signatures. Anyone running GC-MS looks for clean spectra, and small impurities can swamp critical peaks. Stability data gets skewed. Intermediate batches carry forward the burden, making downstream reactions unpredictable. In medicinal chemistry, that risk compounds; impurities might introduce toxicity or throw off biological assays.
Every time a supplier advertises “typical” purity, I pull up publications or regulatory guidelines. For instance, European Pharmacopeia and US FDA both expect full impurity profiles for drug precursors. Gaps appear if suppliers cut corners or skip comprehensive testing.
What Can Buyers and Sellers Do?
People in science should get better at reading between the lines on a spec sheet. Several labs I’ve worked with now require a pre-shipment sample for independent analysis. HPLC, GC-MS, and elemental analysis all generate a more complete picture. If the price looks suspiciously low or there’s no mention of “trace metal analysis,” I turn to a supplier with a longer track record.
Suppliers can win repeat customers by including full spectra and method details with every shipment. Open lines for technical support help as well—nobody wants to operate in the dark, especially on critical projects.
Moving Toward Better Practices
Instead of treating purity as an afterthought, I find more labs document their own checks and maintain an archive for later reference. Government and academic funding bodies would do well to require traceability from raw materials all the way through final results. Joining forces with reputable vendors who back up their numbers just makes sense for the whole field.
For anyone running high-stakes chemistry or biological screening, sweat the details. Purity means fewer surprises, clearer results, and stronger science.
Looking at the Hazards
People tend to treat boronic acids as chemistry shelf-fillers, but 3,5-Difluorophenylboronic Acid isn’t something to brush off. Its molecular formula, C6H5BF2O2, with those two fluorine atoms, points to certain risks you can’t ignore in a working lab. Fluorinated compounds don’t play by the same rules as simple hydrocarbons, so taking shortcuts with storage or PPE creates real trouble.
Unlike regular solvents and organics, boronic acids bring some toxicity and are known to cause respiratory tract irritation. The fluorine atoms don’t just sit quietly. Even small exposures can lead to eye redness, coughing, and in some cases, skin reactions. The fine crystalline powder feels deceptively harmless to the touch, but contact over repeated uses almost guarantees you’ll run into itching or dryness. Inhalation matters most—a quick scoop of an unprotected batch raises dust, and suddenly the whole room has a tickle in the nose.
Why Good Practice Matters
Chemistry labs sometimes let things slide, especially with solids. In everyday work, I’ve seen bottles of this compound sitting open, surrounded by residue. It only takes a light draft or a bit of sweat on your gloves to spread the substance far beyond the bench. Years ago, running Suzuki couplings, no one wanted to deal with the crusty glassware coated in boronic acids. Some just rinsed with cold water, thinking it would be harmless. But these compounds get into sinks, react with mild acids, and the byproducts often raise questions no one wants to answer. Over time, careless habits can add up—leading to headaches, fatigue, or in rare cases, health notices from building management about unexplained chemical smells.
More experienced chemists push for double-layer nitrile gloves, dust masks, and something as basic as taping up reagent jars after use. Flammable risks aren’t the star concern, but heat does break down the boronic acid to messy, sticky gunk that clings to hotplates and occasionally sparks fires when people least suspect. Labs that skip the right ventilation risk building up vapors. Regulators in the US and Europe rate these boronic derivatives as irritants, and Material Safety Data Sheets recommend splash goggles, face shields, and proper lab coats every single time. It’s not bureaucracy at work; nobody wants a classmate or coworker with streaming eyes from a forgotten powder spill.
Steps Toward Safer Handling
Standing by the fume hood, it pays to build habits early. Always weigh it out inside the hood, never on an open bench. Once, I caught myself trying to save time by transferring powder in a hurry, and the next hour brought sneezing I couldn’t stop. That little bit of vigilance—tapping out the powder slowly, keeping jars tightly capped—never wasted time compared with a ruined afternoon. If you spot powder on gloves, switch them before turning to clean glassware or type notes on your laptop. These small steps help everyone keep safe over weeks and years.
Storage also deserves respect. The best labs label the jar date of receipt and track open bottles. Storing it at room temperature away from sunlight fits most protocols, but moisture can eat through seals. Keeping desiccants in the cabinet avoids lumpy, unusable powder and sneaky leaks along shelves, charging the air with tiny, irritating particles. If your lab shares space with synthetic organic chemists and biologists, make sure the cleaning protocol is posted and followed, not just tucked into a binder.
Ultimately, 3,5-Difluorophenylboronic Acid demands the same care as other tricky reagents in modern research. Respecting the hazards makes the work safer, helps the results stay trustworthy, and saves you from slow-building risks that too many people only notice when it’s too late to go back.