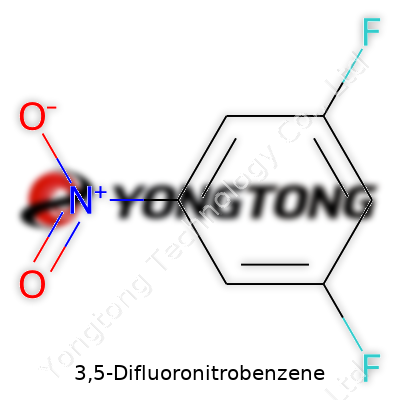
3,5-Difluoronitrobenzene: A Commentary on Evolution, Practice, and Prospects
Historical Development
Early efforts to enhance aromatic chemistry led researchers to explore fluoroarene derivatives throughout the 20th century. 3,5-Difluoronitrobenzene emerged as a compelling compound for both academic and industrial chemists seeking new routes in organic synthesis. Labs in Europe and North America pursued halogenation and nitration techniques by the 1950s, applying innovations in fluorine chemistry that found roots during the synthetic dye revolution. By the 1970s, this compound’s production shifted from basic research to practical intermediates as pharmaceutical and agrochemical sectors demanded reliable building blocks. The steady march of progress in fluoroarene chemistry shows no signs of slowing, with evolving tools now streamlining synthesis and boosting yields without the harsh conditions once considered unavoidable.
Product Overview
3,5-Difluoronitrobenzene delivers a unique combination of properties, making it a choice candidate in labs looking for a stable nitroaromatic bearing fluoro substituents. Sporting a pale yellow tint, it holds form as a crystalline solid under ambient conditions. Unlike more volatile aromatics, the difluoro-nitro combination gives a noticeable edge in both chemical stability and versatility. Few compounds offer such balanced reactivity, which explains its steady presence in catalogs of advanced synthetic intermediates. My time in medicinal chemistry showed that reaching for a bottle of this compound speeds up development cycles, especially when targeting aryl fluorine substitution patterns uncommon in nature yet essential for new drug scaffolds.
Physical & Chemical Properties
Measured at the bench, 3,5-Difluoronitrobenzene reveals a melting point between 40 and 44°C, with a boiling point close to 220°C under atmospheric pressure. Characteristic density (around 1.47 g/cm³) signals manageable handling, and solubility checks confirm it prefers organic solvents such as dichloromethane and acetone. With a molecular formula of C6H3F2NO2 and a molar mass about 159.09 g/mol, the molecule presents well-defined symmetry along its aromatic core. The nitro group draws electrons away, amplifying the withdrawal effect from the two fluorines, making the arene ring less nucleophilic and more attractive in nucleophilic aromatic substitution. Anyone using the compound can see straightaway why these traits support late-stage functionalization in complex synthesis.
Technical Specifications & Labeling
On any product label, you’ll notice purity standards, often holding at 98% or higher, with commonly listed trace impurities such as monofluoronitrobenzenes or related by-products from incomplete halogenation. Storage advice usually restricts direct sunlight and moisture, with recommendations for sealed containers under inert gas. Safety sheets highlight both health and environmental considerations—including irritant labels. The European REACH database flags it as an industrial chemical subject to safety and handling standards. From a working chemist’s point of view, keeping these facts handy matters, especially in regulated settings where batch-to-batch reproducibility secures trust with scale-up partners.
Preparation Method
To synthesize 3,5-Difluoronitrobenzene, most labs opt for a two-step process. Start with difluorobenzene as a precursor, followed by nitration under controlled acid conditions—concentrated sulfuric and nitric acids at carefully moderated temperatures. Stir times and acid ratios make all the difference, and small adjustments in protocol often shift selectivity between ortho, meta, and para isomers. Decades ago, early synthetic routes produced a mishmash of products, complicating purification, but today’s chromatography and crystallization techniques separate this target efficiently. In my own work, swapping in mild nitrating agents reduced hazardous fumes and improved environmental compliance, cutting down the risk to techs and students at the bench.
Chemical Reactions & Modifications
Researchers value 3,5-Difluoronitrobenzene for its ready response in nucleophilic aromatic substitution, especially where the nitro group activates ortho and para positions toward attack. Pounds of it find use every year in reactions introducing amino, alkoxy, or thiol groups, with transition metal catalysis further expanding its range. Hydrogenation transforms the nitro group into an amine, opening the door to new fluorinated anilines—powerful motifs in pharma research. I’ve seen students develop new heterocycles starting from this one nitroarene, discovering that electron-withdrawing fluorines boost their reaction control every step of the way.
Synonyms & Product Names
Across chemical suppliers and regulatory lists, you’ll see names ranging from “1,3-Difluoro-5-nitrobenzene” to “Benzene, 3,5-difluoro-1-nitro-”. The CAS registry cites 2264-31-5 for reference. Trade distributors sometimes abbreviate it to DFNB. Ordering the right product means double-checking each label for isomer purity, as mislabeling plagues even reputable dealers. I’ve seen late project delays because shipments arrived with a mixture of 2,4- and 3,5-isomers, reminding everyone that rigorous verification can’t fall by the wayside.
Safety & Operational Standards
Solid risk control starts with proper safety gear. Nitrated aromatics like 3,5-Difluoronitrobenzene warrant gloves, lab coats, and full fume hoods. Inhalation or skin contact can provoke irritation, and prolonged exposure raises concerns about liver and kidney impact. Waste streams containing this material head straight for hazardous disposal, following local and federal guidelines. Labs using it in scale-up processes train teams not only on accident prevention but on emergency actions for spills or exposure. Schools and companies look to clear labeling and routine audits, both for worker safety and legal compliance, leaving nothing to chance.
Application Area
Industries working with pharmaceuticals rely on 3,5-Difluoronitrobenzene to produce essential fluorinated intermediates for CNS-active drugs and potential anticancer agents. Agrichemical firms exploit its unique structure for synthesizing analogues with greater persistence or selectivity. The electronics sector values fluorinated aromatics for developing liquid crystals and specialty coatings. During my stint with a specialty chemical startup, our team found that the compound’s dual electron-withdrawing groups made it a linchpin in molecular switches, delivering sharp performance gains that off-the-shelf phenyl rings simply couldn’t match. Across each field, the demand for tailored synthetic handles propels ongoing demand.
Research & Development
Chemists in academic and industrial labs keep pushing the boundaries by testing new reaction conditions and catalysts. Modern trends see more green chemistry protocols—employing room-temperature ionic liquids, recyclable catalysts, and minimized waste streams. Biotransformation studies seek to mimic natural enzymes as routes to cleaner conversion. High-throughput screening platforms now automate much of the repetitive work, letting researchers tackle functional group tolerance or chain extension on a far broader scale. Students gain hands-on skills while supporting patent-driven innovation from bench to pilot plant. This hands-on effort ensures a steady pipeline of new fluorinated building blocks poised for fast-moving therapeutic research.
Toxicity Research
The safety profile for 3,5-Difluoronitrobenzene draws scrutiny in both lab-scale and regulatory contexts. Acute exposure data show moderate toxicity by ingestion or skin absorption, prompting clear safety signage in workspaces. Animal studies from the last twenty years detail metabolic breakdown into fluoride ions and nitroaromatic fragments, some of which can stress detoxification pathways in liver and blood. Persistent residues trigger environmental concerns, and responsible labs monitor for bioaccumulation, especially in aquatic discharge. Careful documentation of all health effects helps guide permissible exposure limits and informs workers on risks with each experiment they run. Keeping these records transparent has turned into best practice for research groups and manufacturers alike.
Future Prospects
3,5-Difluoronitrobenzene stands at a crossroads for both classic and emerging applications. As more pharmaceutical and materials research depends on accessible, reliable fluorinated aromatics, the demand won’t slow down. Newer green process technologies—catalytic fluorination, safer nitration, and more selective transformation protocols—promise to cut costs and environmental liabilities. Academics look to chemical informatics and artificial intelligence to predict new derivatives and optimize synthesis at the molecular level. My experience in project-driven research shows that every incremental improvement—whether in safety profile, yield, or reactivity—ripples outward, creating better medicines, more robust agrochemicals, and novel materials for the next decade. Those keeping up with both basic science and engineering advances will shape the market and set the standard for responsible, forward-looking chemistry.
Handling Chemicals Isn’t for the Faint of Heart
Factories that handle 3,5-difluoronitrobenzene usually keep their doors pretty much sealed shut to tourists. This chemical requires careful handling; after all, it belongs to a group of substances that demand respect. Over years spent in industrial and research labs, I’ve seen how getting these materials from barrels to finished molecules isn’t just a technical job—it takes old-fashioned vigilance. Chemicals like this aren’t household names, but they help drive progress in pharma, electronics, and crop science.
Building Block for Pharmaceuticals
Drug makers hunt for the next breakthrough, and molecules like 3,5-difluoronitrobenzene play a behind-the-scenes role. Medicinal chemists use it as a starting point to build more complex structures. The nitro and fluorine groups bring punchy reactivity—letting chemists add, swap, or rearrange functional groups until they land on something that works in the body. This chemical helps launch the development of antivirals, anticancer candidates, and other drugs where a simple swap on the benzene ring changes everything about how it interacts with a disease target. Without this type of intermediate, progress could slow to a crawl.
Pushing Boundaries in Agrochemicals
Farm work and chemistry may not seem to share much, but crop protection leans on innovation at the molecular level. Agrochemical firms start with molecules like 3,5-difluoronitrobenzene because its structure makes it a top choice for switching out fluorines or re-engineering the nitro group. That flexibility means scientists can tune products for greater potency or selectivity. Whether fighting soybean rust or stubborn weeds, effective protection often begins with a synthetic intermediate like this.
Advancing Modern Materials
The electronics industry prizes reliability. Engineers and chemists in this space use 3,5-difluoronitrobenzene as a key ingredient in the quest for better circuit boards and display technologies. Its unique set of double-fluorines and a nitro group modifies polymers so they can survive in tough environments, from the heat of a smartphone to the precision of a medical device. New coatings, adhesives, and advanced fibers frequently trace their origin back to a small set of specialty chemicals, and this molecule tops the list.
Not Just Any Substitute Will Do
It’s tempting to think you could swap out 3,5-difluoronitrobenzene for something less complicated or less expensive. That approach rarely pays off. You gain a unique combination of stability and reactivity with this compound. Trying substitutes could mean missing out on performance or even safety. My experience with trialing alternative syntheses often led to setbacks—right when you thought you’d found a shortcut, some subtle chemistry snag would crop up and cost precious time.
Handling Safety and Looking Ahead
Working with this compound means paying attention. Strict safety procedures must run every hour of every shift. Training isn’t a one-off—successful teams build habits through repetition, whether mixing batches or disposing of waste. At the same time, responsible handling practices give peace of mind to everyone on the floor and in the neighborhoods nearby.
The future promises further demand for precise, reliable intermediates. If you’re building tomorrow’s medicine, a greener pesticide, or a phone that survives drops and spills, you’ll want tools that don’t let you down. Every year, chemists ask themselves: can we go faster, safer, smarter? Chemicals like 3,5-difluoronitrobenzene keep showing up in those answers.
Getting Familiar with 3,5-Difluoronitrobenzene
A compound like 3,5-difluoronitrobenzene doesn’t make headlines unless you’re flipping through a chemistry journal or working inside a lab. Yet, learning about its molecular formula—C6H3F2NO2—reminds us how much everyday life relies on the building blocks of science. Its molecular weight clocks in at 159.09 g/mol, a number that means more than just a footnote on a reference sheet. Years of working around chemical compounds taught me that these numbers often guide entire projects, shape product development, and flag safety risks before a single experiment begins.
Why These Numbers Matter
Formulas and weights don’t just decorate product sheets. Chemists and materials scientists use them to figure out how much product to measure, store, and ship. A single decimal can guide process safety or cause an expensive hiccup in production. I’ve watched teams solve real problems by double-checking a molecular weight before mixing large batches in pharmaceutical manufacturing. Even a small error—say, confusing a structural isomer’s formula—can unravel product quality and result in huge financial setbacks.
Health and Safety: Protecting Workers and Environment
When people handle nitrobenzene derivatives, knowing the molecular formula saves lives. These substances usually present toxicity risks. A slight change in formula sometimes means a new hazard classification. In my own experience, the safety protocols for storing or using 3,5-difluoronitrobenzene rely directly on knowing its exact composition. Lab reports and safety data sheets (SDS) base their recommendations on this detail. Regulators, too, reference the exact chemical profile when setting exposure limits. These numbers become the language that unites scientists, safety managers, and policymakers.
Supporting Responsible Innovation
The development of chemical products, from specialty coatings to pharmaceuticals, counts on accurate formula information. 3,5-difluoronitrobenzene serves as an intermediate for producing advanced molecules. Without firm knowledge of its formula and weight, product developers might hit roadblocks or even introduce waste into a supply chain. Years spent on R&D projects convinced me that tracking every gram and every atom translates directly into cost savings, faster permits, and safer processes. Along the way, companies meet environmental and compliance targets more reliably.
Tackling Chemical Waste and Regulatory Pressures
The days of dumping leftover chemicals or ignoring trace impurities are over. Growing global scrutiny keeps everyone accountable, from research chemists to factory-floor technicians. If a batch of 3,5-difluoronitrobenzene sits off-spec due to a documentation error or confusion over molecular weight, disposal costs climb and audit trails blur. Simple diligence—ensuring everyone understands the compound down to its atomic composition—cuts through regulatory headaches. This has saved teams I worked with hours of back-and-forth during audits.
Solutions Rooted in Accuracy and Education
Investing in chemistry education pays off well beyond the classroom. Making sure lab personnel can read chemical formulas, calculate molecular weights, and grasp downstream implications safeguards every step of the supply chain. Providing easy access to standard references, updated MSDS sheets, and clear communication—this lays the groundwork for safer, smarter operations. Peer review and digital tools can prevent costly slip-ups, a lesson I can vouch for many times over.
The Realities of Chemical Storage
Walking into any chemistry lab, you can spot the difference between a well-managed corner and a hazardous one. Bottles crammed on an open shelf, missing labels, and cracked lids increase the risk, especially with compounds like 3,5-difluoronitrobenzene. This compound, with its sharp nitro scent, fits right into the family of aromatic chemicals with a reputation for toxicity and reactivity. It deserves more attention than just a spot behind the latest delivery of solvents.
Keeping Safety Simple: Temperature and Environment
Any chemist who has experienced the sting or dizziness from volatile aromatics understands that proper temperature control makes a difference. For 3,5-difluoronitrobenzene, a cool, dry spot out of direct light reduces decomposition risk. The ideal is a locked chemical cabinet, near a fume hood, far from heat sources. Solid nitro aromatics handle warmth poorly and start to degrade or give off harmful fumes. Those fumes can build up and create dangerous pressure if left unchecked.
Humidity also causes problems. Moist air adds water to the container, which can trigger unwanted reactions or corrode the container’s cap. Gravelly deposits on the lip or yellow tint around the threads point to improper storage. Keeping desiccant packets nearby, or using a dry box, keeps moisture in check and avoids a sticky, degraded mess.
Choosing the Right Containers
Glass stands up best to fluorinated aromatics. Polyethylene bottles soften over time, so glass, with a tight-fitting lid, keeps out air and moisture while holding up over years of use. Screw caps with PTFE liners work best because they resist corrosion from both nitro and fluorine atoms. Worn-out plastic lids get brittle and flake into the bottle itself—something I caught just once before a spill proved why quality materials matter.
Labeling Isn’t Just Bureaucracy
Sharpie fades. Masked tape peels. A printed, solvent-resistant label with full name, date received, and hazard info tells a new user exactly what’s inside, even months down the line. Too many labs fall into the trap of “I’ll remember what it is.” Even one misplaced sample can mean a dangerous mix-up, especially with similar-looking yellowish crystals found among substituted benzenes.
Handling Spill and Exposure Risks
Spill kits with inert absorbent, like clay or diatomaceous earth, should live close to the storage area. Simple nitrile gloves, splash goggles, and lab coats block small exposures, but splash screens count if the chemical sees regular handling. The sharp, acrid vapor demands serious respect—just a whiff signals it’s time to check the ventilation system and replace the hood filter. Any exposure means immediate washing, not a shrug and a promise to deal with it at lunch.
Minimizing Waste, Protecting the Space
Drip trays under storage racks and chemical-resistant mats catch spills before they creep under equipment. Used vials, pipette tips, and wipes should go straight into sealed waste containers—one slip-up, one dropped piece of glass, and suddenly cleanup becomes a team-wide headache. Unused or expired product ought to follow regulated hazardous waste disposal; pouring it down the drain is a recipe for a landlord phone call and a safety violation.
Staying Ahead with Organization
A little order pays off. Separating strong acids, bases, and oxidizers away from 3,5-difluoronitrobenzene avoids surprise reactions. Built-up dust or mystery residue on the shelf signals maintenance is overdue. Good storage isn’t flashy, but each small effort keeps dangerous incidents from cutting a project short—and ensures everyone gets home safe at the end of the day.
A Chemical That Deserves Respect
Anyone who’s spent time in a lab with aromatic fluorinated compounds knows that even experienced chemists pause before reaching for nitrobenzenes. 3,5-Difluoronitrobenzene isn’t a casual bottle you pop open. It’s got a nitro group, which brings both power and potential for danger. The fluorine atoms give it a sharp edge, literally and figuratively, when it comes to reactivity and health hazards.
The Hazards Don’t Wait for Mistakes
Skin contact causes painful burns, and vapors go through gloves and make their way into your lungs. This stuff is also quite toxic if inhaled or swallowed. Respiratory irritation kicks in fast, often before you realize there’s vapor in the air. I’ve seen a seasoned technician ignore a tiny splash while distracted, and the redness didn’t wait to show up.
Physical chemists know that nitrobenzenes sometimes trigger allergic reactions after repeated exposure, leading to skin sensitization or even chronic issues. Fluorinated organics, as a class, rarely play nice with the environment. A spill doesn’t evaporate and disappear. It sticks around and causes headaches for safety officers.
PPE: More Than a Lab Coat and Gloves
Safety starts before opening the bottle. Nitrile gloves alone don’t stand much of a chance against aggressive organics—upgrade to thicker laminate gloves. Double gloving is common sense. Trade your comfortable cotton lab coat for chemical-resistant or disposable Tyvek. Face shields block splashes, but won’t help with invisible fumes—so a certified cartridge respirator becomes part of your kit, especially in less-ventilated setups.
Checking the fit of goggles and making sure sleeves cover wrists tightly matters a lot more than it sounds. Take the time to snug up every opening on your PPE. That one exposed patch of skin often turns up as the spot of regret after a splash.
Engineering Controls Aren’t Optional
Fume hoods aren’t just for handling highly volatile organics. With 3,5-Difluoronitrobenzene, even brief casual handling belongs under a well-functioning hood. Ventilation matters—facility managers must keep airflow rates tested and filters swapped out at manufacturer-recommended intervals.
Dedicated waste bins lined with compatible materials keep disposal smooth. Open-flame heating sources invite trouble, since nitrocompounds sometimes surprise with their heat sensitivity. Use heating mantles or hot plates with clear temperature cutoffs.
Emergency Protocols and Training
Regular drills help everyone react without hesitation. People freeze during real accidents if emergency showers and eye stations are not clearly mapped out. Written protocols covering minor spills, large-scale emergencies, and decontamination should sit no farther than an arm’s length from the workspace. Printouts on walls work better than digital files buried on a server.
First responders need fast, clear access. Every team member learning the route to safety showers, extinguishers, and cleanup kits can make the difference when seconds count. Medical contacts should be on speed-dial, and an incident logbook can help spot patterns before they turn into real disaster.
Chemistry Is Always a Team Sport
Open, honest communication with lab mates or co-workers keeps everyone sharp. Safe work practices stick best when discussed out loud, not just handed down as protocols. I’ve seen teams catch issues in each other’s setups because talking about “why” beats a checklist mindset. Respect for a compound like 3,5-Difluoronitrobenzene comes from real experience and a willingness to learn from each close call.
Don’t cut corners. Invest in better protective gear, keep the workspace uncluttered, and never take hazardous chemicals out of controlled areas. The chemical doesn’t care about your schedule. It only follows chemistry.
Walking Through Purity in Chemical Supply
Anyone who’s ever worked in a lab—or tried to source chemicals for a project—knows that purity matters, but not always for the same reasons. Take 3,5-difluoronitrobenzene. One lab might require it for pharmaceutical research, pushing for analytical grade because a contaminant can sabotage the whole process. On the other hand, a manufacturer producing bulk intermediates for dyes might accept a technical grade. For buyers, it’s not a one-size-fits-all approach, and availability reflects those demands.
Why Purity Dictates Performance
My early years in a university setting taught me the pain of unreliable reagents. A single batch of material with “unknown” specs derailed a week’s work and wasted valuable grant money. No one wants to discover that the 3,5-difluoronitrobenzene in the fridge contains enough unidentified byproducts to mess with a sensitive reaction. Pharmaceutical research asks for purity above 98%—sometimes referenced as “analytical” or “reagent” grade—because every side component can generate new impurities or unpredictably shift the reaction’s direction.
Manufacturers working at scale, such as those supplying the electronics or dye synthesis space, often choose industrial or technical grades. They’ll accept some upstream impurities if it means a lower cost, so long as those byproducts don’t interfere significantly with the final product. There’s a tradeoff between price and precision, often balancing on decades of know-how or a deep understanding of their own process limitations.
Knowing What’s in the Bottle
Chemical catalogs from major suppliers illustrate the differences clearly. Some offer data sheets specifying everything from melting point to impurity profiles. Walk through any trade show in the chemical industry, and you’ll overhear conversations about batch-to-batch consistency, how easy it is to trace the lot, and certificates of analysis. These are the sort of quality assurances researchers and manufacturers rely on to keep their processes predictable.
A quick glance through publicly available safety data sheets shows that 3,5-difluoronitrobenzene comes in multiple grades. You’ll see 95% or 97% listed for industrial applications, and pure, labeled for analytical needs, reaching above 99%. The price differential isn’t subtle. A half-liter bottle at 99% costs much more than the technical-grade equivalent, but for some, that’s the price of certainty.
Challenges and Solutions
Not every supplier carries every grade. Supply can dry up for the highest purities, and smaller labs in regions with less access to international distributors sometimes go without. Based on past experience, ordering from credible sources with up-to-date data sheets can keep surprises to a minimum. Investing in quality control testing, even with a portable spectrometer or a quick TLC, catches errors early before anyone spends days troubleshooting an avoidable mistake.
Open communication with suppliers makes a difference. Requesting a certificate of analysis for each lot, discussing special needs regarding solvents or trace metals, and verifying the logistics chain—these small steps strengthen reliability. Companies that audit their sources or create long-term relationships have a much smoother ride, sidestepping those “out-of-spec” disasters that sneak up when sourcing from unfamiliar agents.
Supporting Research, Protecting Results
Without careful oversight, a small impurity snowballs into a big headache. Consistently sourcing the correct purity of 3,5-difluoronitrobenzene separates successful projects from those marred with unexplained results. Personal experience in group research, combined with years of watching projects fly or fail based on simple sourcing, paints a clear picture. Purity doesn’t just influence reactions—it can make or break both reputations and budgets.