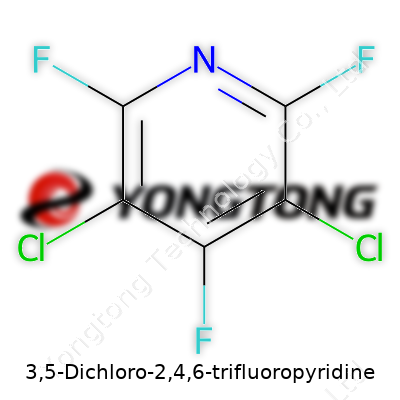
3,5-Dichloro-2,4,6-trifluoropyridine: In-Depth Look
Historical Development
The story of 3,5-Dichloro-2,4,6-trifluoropyridine traces back to the post-World War II boom in organofluorine chemistry. Scientists ramped up the development of halogenated pyridines in the 1950s and 60s, searching for molecules with powerful reactivity and stability for diverse uses. Research groups at industrial giants and public universities zeroed in on multi-halogenated aromatic systems for their potent electronic properties. As more complex agrochemicals and pharmaceuticals came into the market, fine chemicals like 3,5-Dichloro-2,4,6-trifluoropyridine grew important. While the compound didn’t make headlines like DDT or penicillin, it carved out a niche in specialty synthesis. Chemists tinkered with approaches, balancing availability of feedstock materials like 2,4,6-trifluoropyridine, and developed improved chlorination protocols with lower environmental impact. Today’s manufacturers stand on decades of trial-and-error methods that pushed efficiency and purity higher, while gradually finding routes that minimize toxic byproducts and safeguard worker health.
Product Overview
3,5-Dichloro-2,4,6-trifluoropyridine joins a specialized family of halogenated pyridines recognized for their agile reactivity. What sets it apart in the catalog is its trifluorinated backbone combined with two chlorine atoms at the 3 and 5 positions. Chemists value this arrangement for building more complex molecules, especially where both fluorine and chlorine functionality matter. Sold in a range of pack sizes, the product typically comes as a colorless to pale yellow liquid, packaged in airtight glass or PTFE-coated containers. Companies supplying research labs or pharmaceutical companies ensure trace levels of moisture and metal ions sit well below industry thresholds, and offer authentication through analysis certificates detailing exact composition and impurity profile. This precision helps researchers avoid costly surprises, making the chemical a reassuring choice for demanding tasks.
Physical & Chemical Properties
Examining 3,5-Dichloro-2,4,6-trifluoropyridine under the lens of basic chemistry, the molecule weighs in at about 235 g/mol. In the lab, you’ll notice it gives off a sharp, almost medicinal odor. It boils at approximately 142 °C and melts below freezing, around -10 °C. Its density clocks in above water, settling near 1.6 g/cm³. The combination of fluoro and chloro substituents toughens the molecule, so it shrugs off weak acids and bases, though it can succumb to energetic nucleophiles at elevated temperatures. Poor solubility in water turns into ready mixing in common organics such as dichloromethane, acetonitrile or DMF. Storage conditions demand a dry, cool environment. Light and humidity speed up degradation, catalyzing slow hydrolysis or even rearomatization in extreme cases. Experienced chemists recognize that fluorinated pyridines push glassware and seals to their limits, so careful selection of handling materials pays off.
Technical Specifications & Labeling
Producers stick close to established technical standards when shipping this compound. You’ll find labels listing its formula (C5Cl2F3N), CAS number (though keep in mind, regulations or local conventions can shift these slightly), and key hazard warnings—eye, skin, and respiratory irritant at a minimum. Material Safety Data Sheets give explicit directions on accidental release and safe disposal, and lot-specific certificates usually report purity at 98 percent or better, with side notes on water content and identified minor isomers. Some suppliers run extra analytics, like NMR and GC-MS, to guarantee structure and check for trace metal or halogen contamination—essential for pharmaceutical precursors or electronics research. Rigorous labeling and batch tracking simplify troubleshooting and compliance audits in high-stakes manufacturing environments.
Preparation Method
Making 3,5-Dichloro-2,4,6-trifluoropyridine takes finesse, since the starting materials themselves tend to be volatile and sometimes hard to source. Industrial synthesis usually begins with 2,4,6-trifluoropyridine, exploiting the fact that its remaining positions are primed for targeted halogenation. Chlorination routes split between direct treatment with chlorine gas in the presence of Lewis acids and stepwise introduction using N-chlorosuccinimide or phosphorus pentachloride. Temperature control and slow addition rates prevent undesired over-chlorination or ring degradation. Purification relies heavily on distillation and liquid-liquid extraction to weed out polyhalogenated byproducts, followed by column chromatography for the most demanding applications. As environmental regulations tighten, chemists have tweaked these protocols to recover solvents and capture off-gassing halogen acids, using scrubbers and sealed systems to protect staff and minimize neighborhood impacts.
Chemical Reactions & Modifications
The dichloro-trifluoro motif of this pyridine lays out a tempting menu for synthetic chemists. Electron-withdrawing halogens activate the ring for selective nucleophilic aromatic substitution, especially at positions bearing chlorine. Lab teams have swapped these chlorines with anilines, alkoxides, and even thiols, creating intermediates for custom regulators of biological or physical properties. The fluorine atoms resist most substitution, but open doors for challenging metal-catalyzed couplings or eventual reduction, bringing in new flavors of structural diversity. In my own experience, careful manipulation under inert atmosphere lets one control substitution patterns and steer away from intractable polymeric byproducts that can foul reaction vessels. As methods grow more elegant, fewer steps and less waste define the modern approach to modification, keeping labs safer and cleaner.
Synonyms & Product Names
While most catalog entries use 3,5-Dichloro-2,4,6-trifluoropyridine as the main handle, technical bulletins list alternates such as 2,4,6-Trifluoro-3,5-dichloropyridine and its registry code. Some older texts refer to it under trade abbreviations or development codes, echoing its origin in proprietary agrochemical and specialty chemical programs. These synonyms speak to globalization—each market or company finds names that fit local language and legal nuances. Reliable producers always tie these synonyms back to unambiguous registry numbers, so buyers and regulators alike can match product to standard documentation and avoid missteps in shipping or customs.
Safety & Operational Standards
Safety conversations around 3,5-Dichloro-2,4,6-trifluoropyridine hinge on its toxic potential if mishandled. It irritates mucous membranes and can trigger breathing issues with prolonged or unprotected exposure. Given its volatility and solubility profile, even seasoned chemists double-layer gloves and shuffle work into chemical fume hoods. Emergency gear—eyewash stations, spill kits, and plenty of ventilated workspace—form the backbone of routine lab practice. Responsible producers follow protocols that extend beyond the letter of safety law—routine air sampling, exposure monitoring, and regular training for new hires keep risk dialed down. Waste management steps, from neutralization tanks to secure incineration, keep hazardous residues off the street and out of groundwater. Speaking personally, every incident report I’ve read underscores the importance of up-to-date training, gear audits, and clear labeling to avoid emergencies.
Application Area
This molecule sees most of its action as a building block in pharmaceutical R&D, crop-protection studies, and advanced material syntheses. Medicinal chemists use its halogenated ring to explore lead compounds for antiviral and anti-inflammatory agents, since halogens boost membrane penetration and metabolic stability. Agrochemical designers try out derivatives as next-generation fungicides and herbicides, banking on the unique reactivity enabled by the chlorine and fluorine tags. Electronics and materials researchers find space to investigate it as a monomer or doping agent in specialized polymer films. These practical applications rest on decades of collaboration with downstream partners to troubleshoot performance and regulatory approval hurdles, calling for a nimble approach to quality assurance and documentation.
Research & Development
R&D around 3,5-Dichloro-2,4,6-trifluoropyridine tracks both incremental improvement and moonshot ambitions. Medicinal chemistry teams push methods to modify the molecule’s core more cleanly, while process engineers design greener, scalable manufacturing. Analytical chemists keep busy developing detection methods to monitor trace levels in environmental and product safety surveys. Some labs investigate new biosynthetic mimics and safer alternatives in a bid to avoid hazardous halogenation in plants or bacteria. Strategic collaborations with universities feed a steady stream of new ideas into patent applications and pipeline molecules. I’ve seen grant agencies increasingly fund safer synthesis and lifecycle analysis, nudging companies and academia to focus on cost, safety, and environmental benchmarks in tandem.
Toxicity Research
Toxicology data on 3,5-Dichloro-2,4,6-trifluoropyridine remains a focus given current regulatory momentum. Standard rodent assays show moderate acute and chronic toxicity, with primary targets in liver and kidney tissue. Risk assessments flag inhalation and dermal contact as primary exposure routes. Cell culture studies point to DNA damage at high concentrations, though at industrial concentrations, proper handling limits exposure risk. Environmental studies show persistence in soil and water, driven by the sturdy fluoro ring, so disposal rules reflect these dangers. Experiences in lab and plant environments highlight how proper ventilation, rigorous PPE, and thoughtful waste management shrink both occupational and ecological risk. Policy bodies push companies to share new findings and build transparent databases, letting workers, communities, and regulators respond quickly if a problem emerges.
Future Prospects
Looking forward, the fate of 3,5-Dichloro-2,4,6-trifluoropyridine depends on how industry and regulators embrace safer chemistry. Rising demand in pharmaceuticals, agrochemicals, and niche electronics puts pressure on supply chains to keep up with tight safety and quality standards. Process improvements could trim hazardous waste and boost yields, giving manufacturers a competitive edge. Advances in green chemistry might open doors to catalytic or biosynthetic production routes that sidestep hazardous reagents, slashing carbon footprints and operational risks. Long-term, investment in safer analogs or functional substitutes sits on the horizon, especially as consumer and regulatory demands for transparency and sustainability climb. Researchers stay tuned to societal expectations and changing regulations, weaving responsible manufacturing, ongoing toxicity screening, and nimble market adaptation into the lifecycle of this complex but essential chemical.
Understanding the Formula
Let’s talk chemistry. The chemical formula for 3,5-Dichloro-2,4,6-trifluoropyridine is C5Cl2F3N. You’ve got five carbon atoms, a pair of chlorine atoms, three fluorine atoms, and a nitrogen built around a pyridine ring. The way those atoms line up on the ring changes quite a bit about how the molecule acts in real life.
Why This Molecule Gets Attention
This compound won’t ring bells for most folks, but it deserves more than a skim. It belongs among a family of halogenated pyridines—the kind of stuff chemical researchers and pharmaceutical developers keep close. Modified pyridine rings like this one show up as building blocks in crop protection, specialty additives, and advanced pharmaceuticals.
Adding chlorine and fluorine to the ring isn’t just for show. These tweaks make the molecule tough against breakdown. For instance, most fluorinated compounds resist heat, light, and reactions with other chemicals far better than their simpler cousins. This brings up a tricky trade-off: durability often means persistence in the environment. In fact, halogenated chemicals can stick around for years if released, like what we’ve seen with PFAS and their cousins. Their stability may give big wins in product durability, but these same features often spell trouble for rivers, soil, and even food chains.
Risks and Responsibilities
It’s easy to look at a chemical formula and shrug, but behind those symbols there’s a story with direct influence on food, water, and health. In labs, strict guidelines shape how researchers and plant operators handle chemicals like this. Every factory worker, chemist, and regulator shares a responsibility for keeping these molecules where they belong. Studies by environmental groups and regulatory agencies have shown how small leaks or careless disposal can escalate into bigger problems. Putting proper safeguards in place isn’t a burden—it’s a duty owed to public health and the planet.
Improving the System
Looking for better handling starts with education. Training workers to respect the risks while handling chemicals keeps everyone safer. Manufacturers can invest in containment, use closed systems, and keep waste disposal under close watch with frequent checks and automatic monitoring. Communication also cuts down on mishaps. Good data tracking within facilities and open reporting to safety agencies can help catch leaks or spills before they become disasters.
One promising trend brings green chemistry into research. Chemists look for non-halogenated compounds or design molecules that break down faster in the environment but still do the job. This puts less pressure on recycling or long-term storage. Demand for these greener options pushes the market toward safer formulas without sacrificing performance, which often appeals not just to regulators but investors and consumers too.
Staying Ahead of the Curve
Every time chemistry books add a new formula, teams behind the scenes rethink the full impact from the start. Regulatory agencies around the world keep listing new chemicals for review, and what feels minor today might show up in water or air quality reports down the road. That’s why staying updated—monitoring case studies, talking with researchers, and supporting better tech—benefits everyone. The formula C5Cl2F3N might look like a tongue-twister, but it carries real consequences for science and daily life.
Why This Compound Matters
My first encounter with 3,5-Dichloro-2,4,6-trifluoropyridine came at a mid-sized crop science company juggling product formulas for the next big herbicide. You won’t find flashy headlines about this compound, but for folks in agrochemical labs and pharmaceutical research, it holds a special spot. Put simply, its unique mix of chlorine and fluorine on the pyridine ring offers chemists exciting tools for building up complex molecules.
Agriculture Leans Heavily on It
Most of the world’s food producers wrestle weeds and tough plant diseases each season. Here’s where this tricky little pyridine helps. Its chemical skeleton gets plugged into formulas that bring new herbicides and fungicides to life. Chemists can swap in and out parts of the molecule to control how fast it degrades in soil or how tightly it latches onto plant enzymes. Farmers chasing higher yields end up relying on these breakthroughs more than they realize. According to the Food and Agriculture Organization, up to 40% of crops worldwide get lost to pests and disease, even with all our modern protections. Molecules like this pyridine keep more food out of the compost pile.
Driving Pharmaceutical Research
Drug companies run experiments on thousands of molecules a year, looking for the next pill or injectable treatment. The trifluorinated and chlorinated structure of this pyridine makes it a kind of “starter block” in some projects—sort of like buying a half-built LEGO set. Scientists use it as a scaffold, then attach different chemical groups to tailor a drug’s performance in the body. In cancer therapies and antiviral research, pyridine rings pop up in many success stories. A few reports from respected journals like Journal of Medicinal Chemistry confirm that adding fluorine atoms can help new medicine last longer in the blood and reach hidden corners of the body. This boosts the chance of smaller, safer doses down the road.
Steps Toward Safer Chemistry
There’s a catch, of course. With exotic fluorinated compounds, it pays to think about how they break down once a field or bloodstream has seen them. Older herbicides built on similar rings sometimes stuck around too long, affecting water and wildlife. People in the chemical industry now put more focus on how to break down these molecules safely or choose alternative routes for less persistent residues. Some scientists test “greener” catalysts and design molecules that hold their shape just long enough to be useful, then fall apart into something harmless. A 2023 review in Chemosphere argued that smart design early in the lab can make a difference before tons of product hit the market.
Training, Testing, and Responsibility
Anyone who’s handled specialty chemicals knows protective gear means more than just following regulations—it can mean going home healthy, every time. Labs using trifluoropyridines invest in fume hoods, regular employee safety training, and databases of updated toxicology data. The American Chemical Society warns against overconfidence with any heavily substituted pyridine. Staying informed and following evidence-based guidance helps keep research, production, and application as safe as possible for workers, neighbors, and consumers alike.
Moving Forward
Engineers and chemists solve problems with building blocks like 3,5-Dichloro-2,4,6-trifluoropyridine every day. Whether it winds up in a crop treatment, a drug trial, or a new experiment, its most important job lies in quietly supporting progress while scientists seek better ways to protect health and the environment.
Understanding What’s at Stake
3,5-Dichloro-2,4,6-trifluoropyridine stands out as a chemical with bite. In my time around industrial labs and chemical storerooms, some substances draw respect for more than just their smell or reputation. This one deserves careful attention, between its reactivity and potential to irritate skin, eyes, or lungs. Nobody wants to discover firsthand how quickly things can go wrong because a bottle landed on a dusty, forgotten shelf or a technician grabbed a lab coat stained from last week.
Safe Storage: Keep the Chaos in Check
Chemicals with halogens and fluorine stick around—literally and metaphorically. Here, experts agree: a cool, locked cabinet with solid ventilation helps avoid mistakes. No one likes surprise fumes, and many facilities labor under strict fire code and OSHA rules for a reason. I once worked at a plant where a whiff escaping from a cracked container set off alarms and forced an expensive evacuation. Vapors don’t care about badge access.
Dedicated rooms with negative airflow, chemical-rated bins, and consistent labeling can make or break a quick response. Store this particular pyridine away from acids, bases, and oxidizers—old habits of lumping “lab stuff” in one corner usually lead to trouble. Water, especially humidity, sets off unwanted reactions. Dry conditions go a long way. Pack containers tight, with firm seals, on stable surfaces. In some spots, I’ve seen dry boxes or large desiccators pressed into service just to hold small bottles of trifluorinated intermediates.
Handling: People Matter Most
This isn’t the stuff to splash around. I learned to respect this kind of molecule after watching a colleague develop skin irritation, even though he thought gloves would protect him completely. Nitrile gloves, face shields, and lab coats aren’t for show—this compound wicks through latex faster than you’d guess. Spill kits with activated charcoal or calcium oxide can save time during a slip or drip. Fume hoods aren’t optional; good ones pull stray vapors right out of the picture.
Every time our team went through handling protocols, the message echoed—know the emergency contacts, keep eyewash stations clear, and rehearse what to do if something lands anywhere it shouldn’t. Simple checklists taped above workbenches helped us steer around absent-minded risks. Consider training refreshers not a box to tick but the difference between a safe day and a mess nobody wants.
Solutions: Making the Workplace Safer
Seasoned chemists recommend not keeping more on hand than a project needs. This limits exposure, cuts down fire risk, and helps with paperwork. I’ve seen good results from using smaller bottles with color-coded caps—in group labs, it makes a real difference. Digital inventory logs cut confusion fast when every shift has two or three people clocking in and out.
Regulations may set the minimum, but a shared attitude of vigilance gives every worker peace of mind. Mistakes happen, shortcuts tempt tired hands, and safety habits only stick if management backs them with training, resources, and open communication. In my experience, the best-run labs create a norm where speaking up about possible hazards never feels awkward. Complacency sneaks up when people see “routine” chemical names and forget how unpredictable some molecules get under stress.
Good storage and careful handling don’t just tick legal boxes. They save nerves, skin, and sometimes health. A little extra care pays back everyone in the room, long after the bottle’s empty or the project’s done.
Why This Chemical Matters
Talking about chemicals like 3,5-Dichloro-2,4,6-trifluoropyridine doesn’t usually hit the headlines, but that doesn’t mean the risks fade away in a lab corner. This compound often finds use in specialty chemical industries and pharmaceuticals. The appeal comes from its reactivity and the things it can build, but risks tag along in the shadows. Anyone who’s ever worked with halogenated organic compounds knows that just because a compound isn’t well-known doesn’t mean you can turn a blind eye to safety. Based on my own years working near chemical facilities, complacency causes problems more often than the substances themselves.
Watching What We Breathe and Touch
Let’s focus on exposure, because that’s where the trouble usually begins. Fluorinated and chlorinated pyridines deserve close respect. We know that chlorine and fluorine atoms bring extra hazards, from irritant vapors to lifecycle toxicity. 3,5-Dichloro-2,4,6-trifluoropyridine can give off pungent fumes; inhaling these in an unventilated area provokes coughing, burning sensations, and sometimes, real damage to the upper respiratory tract. Eyes and skin take a hit too, with redness and irritation showing up from even small splashes.
It’s tempting to brush off chemicals like this one with a “standard care should cover it” mentality, though the facts don’t cooperate. Pyridine derivatives have a track record for neurotoxicity, especially at higher exposure levels. Add halogens into the mix, and long-term exposure becomes a bigger puzzle: cumulative organ damage, chronic headaches, and maybe higher cancer risks. Published animal studies point toward these effects, and regulatory bodies, including the NIOSH and EU Chemicals Agency, flag similar concerns for close relatives of this compound.
Handling and Waste: Not Just a Lab Problem
The danger doesn’t end with personal exposure. Disposal brings its own risks. Improper handling pushed halogenated pyridines into groundwater in several incidents worldwide, with cleanup dragging on for years. We know these compounds break down slowly in nature and tend to bioaccumulate. From manufacturing floors to transport trucks, every step can become a hazard if simple best practices slip. In my time volunteering with community environmental groups, we’ve watched how even trace pollution lingers in water and soil near chemical plants.
Building a Safer Approach
Hazard doesn’t equal inevitable harm, but it does call for respect. Engineering controls—like local exhausts and glove boxes—make a difference. Personal protective equipment protects workers, but only when they believe in its importance and use it every day. Companies that train their people, not just once but in regular refreshers, see fewer accidents. Tight controls on labeling, recordkeeping, and emergency response fill in the gaps left by human error and freak accidents.
Solving the larger issue means shifting industry culture. Transparent safety reporting lets everyone know where leaks, exposures, and minor injuries happen, so patterns don’t hide until someone ends up in the hospital. Regulators need to press for full risk disclosure and real toxicity testing on specialty chemicals, not just base ingredients. Everyone benefits from that kind of honesty, from chemical engineers to neighbors living downwind.
Responsible Innovation Over Risk Blindness
No scientific advance justifies ignoring the cost to human health or the environment. 3,5-Dichloro-2,4,6-trifluoropyridine, like many specialty chemicals, tests our ability to manage short-term benefits with long-term responsibility. I believe refusing to cut corners means fewer headlines about disaster, and fewer quietly suffering communities paying for our progress.
High Purity: An Expectation, Not a Luxury
In chemical manufacturing, purity isn’t just a checkbox—it drives every result that matters, from safe downstream synthesis to consistent performance in research. For 3,5-Dichloro-2,4,6-trifluoropyridine, the numbers most researchers want start at 98% and go even higher. Lab supply catalogs and industry suppliers almost always list this compound with a purity of at least 98%. That’s not just for show. Lower-purity material can mess up experiments, introduce question marks in results, or compromise a whole batch downstream. Anyone who’s found a contaminant at the worst possible time knows how quickly that “fraction of a percent” can turn into a mountain of troubleshooting and lost time.
Leaving impurities unchecked may leave you with unusable samples or create safety risks, since reagents and by-products interact with more than just your target process. Chemical analysis results from several manufacturers show impurities—such as unreacted chlorinated or fluorinated pyridines—creeping in at lower purities. That threatens quality for everything built on top of that chain. Analytical certificates and batch traceability don’t guarantee anything on their own, but they tell buyers the supplier takes serious steps to ensure honest reporting and reproducibility.
Packaging: More than a Container
Over years in the field, I’ve unboxed lifesaving drugs in glass vials, bulk nutrients in drums, and specialty intermediates in plastic-lined cans. For 3,5-Dichloro-2,4,6-trifluoropyridine—volatile, reactive, and somewhat niche—packaging isn’t an afterthought. It keeps the product stable, meets regulatory standards, and avoids cross-contamination.
Most suppliers offer this compound in amber glass bottles from 25 grams to 250 grams for lab-scale projects. Larger demands—say, for kilogram-level synthesis—come in high-density polyethylene (HDPE) containers or metal drums with tamper-evident seals and liners designed for chemical compatibility. Glass shields from light, keeping photodegradation at bay, while plastic drums prevent accidental breakage in transit. For anything above personal use, tamper-proof packaging is non-negotiable. Poorly sealed or incorrectly labeled packaging once left a Ph.D. colleague in a lurch, sorting out trace water damage to a sensitive halogenated compound. Just a little moisture or oxygen seepage ruined weeks of setup. This is why buyers in pharma and electronics keep a sharp eye on the packaging material, cap seal integrity, and inert atmosphere labeling.
Bridging Purity and Packaging with Trust and Transparency
Years of dealing with specialty chemicals taught me to dig beneath the surface. I look for suppliers that share full lot analysis, disclose storage histories, and explain how they handle trace contaminants. There’s a world of difference between “greater than 98%” on a data sheet and a complete gas chromatography readout. Seeing environmental handling protocols for sensitive goods gives confidence that the product will match expectations—not just out of the box, but at the benchtop or reactor.
Efforts to improve distribution could center around QR-coded tracking for every lot, rigorous secondary containment, and clear temperature controls. Dangerous materials lose their bite when handlers know exactly what they’re dealing with at every step.
Picking the right grade of 3,5-Dichloro-2,4,6-trifluoropyridine and the right package isn’t just a detail—it’s the first honest step toward high-quality, reliable science or manufacturing. Ignoring the details brings needless risk and—speaking from experience—can blow up more than just the lab budget.