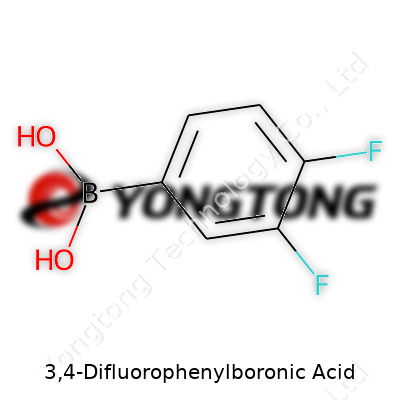
3,4-Difluorophenylboronic Acid: Perspectives on a Key Synthetic Building Block
Historical Development
Back in the mid-20th century, chemists looking to expand the tools for constructing complex organic molecules zeroed in on organoboron compounds. As palladium-catalyzed cross-coupling reactions took off in the late 1970s and 80s, boronic acids, especially substituted phenylboronic acids like 3,4-difluorophenylboronic acid, gained attention. The Suzuki-Miyaura coupling transformed this field, letting researchers add small, functionalized aromatics to their libraries without harsh conditions. Over the years, demand for fluorinated building blocks pushed the development of more efficient syntheses for 3,4-difluorophenylboronic acid, making it a staple in both academic labs and pharmaceutical research settings.
Product Overview
Easy to recognize in a catalog by its sharp white appearance, 3,4-difluorophenylboronic acid often gets tagged with the CAS number 146137-80-6. Chemists like it because it offers a good blend of stability and reactivity inside the bottle, and each batch lands with tight quality specs. Compared to the non-fluorinated analog, the double fluorine substitution often brings new twists to the reaction possibilities, making it more than just a niche item.
Physical & Chemical Properties
Holding a molecular formula of C6H5B F2O2, this compound typically appears as a crystalline solid or fine powder. Its melting point hovers in the 150 to 160 °C range—well-suited for many solid-state manipulations. The boronic acid group at one end readily takes part in hydrogen bonding and forms tight complexes with diols, which can shift its behavior in solution. With two electronegative fluorine atoms on the aromatic ring, the electron density changes, altering its reactivity in cross-coupling and making the product more resistant to metabolic breakdown. In practical terms, it dissolves in polar organic solvents like DMSO, DMF, and to some extent in methanol or ethanol. Water solubility stays on the lower side, which influences how people handle reaction work-ups or extractions after synthesis.
Technical Specifications & Labeling
Product labels usually provide purity, which for research grade hovers above 98%. Moisture sensitivity sits as a minor concern, yet the stable solid form allows storage at room temperature if kept dry. Analytical certificates tend to document not only NMR and HPLC traces but sometimes elemental analysis or residual solvent levels. The packaging almost always includes handling advice because dust from boronic acids can act as a mild irritant if inhaled or if it contacts the eyes. Each bottle also should list batch number and manufacturing date, since trace decomposition may influence reactivity after long storage.
Preparation Method
Organic chemists favor two routes: direct borylation or halogen-lithium exchange followed by quenching with a trialkyl borate or boronic ester. The halogen-lithium exchange starts from 3,4-difluorobromobenzene, which reacts cold with n-butyllithium in a dry ether or THF, forming the lithium salt. A subsequent quench with trimethyl borate at -78 °C gives the boronic ester, which then gets hydrolyzed to the free acid under mild acidic conditions. Recent decades brought in palladium- or copper-catalyzed direct C-H activation, which uses the difluorobenzene directly, lowering waste and speeding up overall synthesis. Industrial-scale preparation leans toward the methods that generate the least hazardous by-products, aided by continuous process optimizations.
Chemical Reactions & Modifications
People see this boronic acid as a reliable partner in Suzuki-Miyaura cross-coupling, using it to introduce a 3,4-difluorophenyl ring into larger aromatic or heterocyclic frameworks. Usually, a palladium catalyst—often with phosphine ligands—brings the coupling to life, letting chemists build biaryls under mild, base-catalyzed conditions. Beyond Suzuki couplings, boronic acids participate in Chan-Lam couplings to form new C–N, C–O, or C–S bonds, especially popular for adjusting pharmacological profiles of small molecules. In carbohydrate chemistry, 3,4-difluorophenylboronic acid can serve as a mediator or sensor component thanks to its reversible binding with diols. Because the boronic acid group itself can be protected or esterified, further downstream modifications expand the toolkit available for complex target synthesis.
Synonyms & Product Names
In chemical listings, this molecule might show up as 3,4-difluorobenzeneboronic acid, m,difluorophenylboronic acid, or with foreign language equivalents in non-English catalogs. Commercial suppliers sometimes use their own coding, yet the CAS number 146137-80-6 remains consistent for ordering or regulatory reference. Scientists searching literature or regulatory documents must watch for the full spread of synonyms to avoid missing key information.
Safety & Operational Standards
Laboratory safety sheets for 3,4-difluorophenylboronic acid mark it as a low-to-moderate toxicity solid, not acutely dangerous but worth handling with the eye protection and gloves standard for organics. While it does not pose the flammability of many solvents, the fine powder form can cause mild respiratory or mucous membrane irritation. If heated to decomposition, traces of boron oxides or fluorinated aromatics may develop; fume hoods keep vapors away from users in these scenarios. Clean-up usually involves vacuuming dust with HEPA filters and avoiding contact with water-sensitive reagents, as hydrolysis sometimes generates boric acid. Chemical waste procedures treat any spent material as hazardous organic solid, bagging it for collection by a licensed disposal service.
Application Area
Drug discovery platforms, agrochemical invention, and functional materials research all draw regularly from stocks of 3,4-difluorophenylboronic acid. Medicinal chemists value fluorinated aromatics since fluorine’s presence often heightens metabolic stability and alters binding affinity without adding bulk. Blockbuster drugs targeting kinases, GPCRs, and proteases sport difluorophenyl motifs as part of their pharmacophores, and the boronic acid building block gives access to customized analogs with a minimum investment of labor. The electronics sector taps into the world of small-molecule semiconductors, where incorporating difluorophenyl moieties in an organic backbone improves charge mobility or environmental resistance.
Research & Development
Innovation in organoboron chemistry leans on reliable building blocks like this one. Ongoing research keeps refining reaction conditions so that even difficult substrates will give high yields and straightforward isolations. In silico docking studies often bring up difluorophenyl-containing candidates, so compound libraries need a steady supply of this reagent. Teams in green chemistry have started looking for ways to recycle spent boronic acids or shift to aqueous systems, reducing both solvent use and cost. Meanwhile process chemists work on refining the catalysts and ligands used in coupling reactions, sometimes cutting into the price and making these syntheses accessible for scaled-up manufacturing rather than just gram-scale experiments.
Toxicity Research
So far, animal studies and environmental toxicity screenings rate 3,4-difluorophenylboronic acid as fairly low in acute toxicity compared to heavy-metal or halogenated solvents, but questions linger around chronic exposure and environmental breakdown products. Trace amounts often escape into water or waste streams during production, and regulatory groups keep a close eye on how these boronic acids and their residues interact with aquatic organisms or microorganisms in wastewater treatment. Proper containment measures during handling and disposal lower the real-world impact. In cell-based experiments, moderate concentrations can cause stress, but no strong evidence yet points to bioaccumulation or long-term risks at current usage levels. For pharmaceutical end-products, the parent boronic acid rarely persists, since the aromatic group is coupled and transformed in the synthetic route.
Future Prospects
Everything about fluorinated building blocks points to rising demand, driven both by new molecular designs in pharmaceuticals and the widening adoption of modular synthesis. As automation and AI-driven drug design keep evolving, commodity chemicals like 3,4-difluorophenylboronic acid serve as the backbone of high-throughput screening libraries. On the regulatory side, tighter restrictions on persistent environmental chemicals may guide improvements in purification and waste management practices. Supply-chain diversity—including greener reaction processes and regional manufacturers—looks set to boost both accessibility and sustainability. In a world where every molecular tweak can open a new drug or material, reliable access to well-characterized building blocks gives scientists a head start on tomorrow’s discoveries.
Connecting Chemistry to Medicine
Ask anyone working in drug development about boronic acids, and you’ll likely hear stories about their steady role as building blocks. 3,4-Difluorophenylboronic acid gets a lot of attention because it helps in making small molecules act the way chemists want. In the search for effective medicines, especially to treat chronic illnesses and cancer, chemists don’t have time for unnecessary steps or guesswork. The di-fluoro substitution on the phenyl ring gives researchers control over drug properties, like how well a new treatment dissolves or how long it sticks around in the body.
Look at the waves made by new kinase inhibitors, which help slow down cancer cell growth. Many of these drugs rely on constructing their core skeletons using Suzuki–Miyaura cross-coupling reactions, and that’s where this acid pulls its weight. The 3,4-difluoro pattern on the ring influences both activity and selectivity, steering drug candidates toward fewer side effects. The need for these fine-tuned intermediates isn’t just a trend: reports in journals like the Journal of Medicinal Chemistry keep growing, reflecting what’s happening in real companies and not just academic labs.
Material Science Turns to Smart Chemistry
Materials scientists also take notice. Modern electronics don’t rest on copper alone. OLED display makers turn to boronic acid derivatives to build specialty polymers, looking for sharp control over how light travels through organic films. Small tweaks to the aromatic ring, such as adding fluorine, lead to improvements in brightness and energy efficiency. That means brighter screens on phones and lower power consumption, both priorities as gadgets get smaller and faster. Fluorinated boronic acids help bridge the gap between chemistry and engineering—real change starts at the molecular level.
3,4-Difluorophenylboronic acid fits into this as a starting block, letting researchers connect it with other aromatic partners. These links have a direct effect on thermal stability and electronic properties. New reports from university collaborations show that switching out hydrogen for fluorine atoms isn’t just for show—devices tolerate higher temperatures and stand up better to stress, meaning fewer breakdowns and wasted resources.
Unavoidable in Modern Synthesis
In the lab, synthetic chemists treat 3,4-difluorophenylboronic acid as a staple for making diverse molecules. Its reliable reactivity trims downtime and cuts out tedious purification. If you walk into a graduate student’s fume hood, you’ll see a bottle of it next to the rest of the boronic acids. Cost isn’t minor, but suppliers and catalog companies keep bringing prices down as demand climbs. As patents expire and competition heats up, the acid becomes more accessible to both small startups and big drug companies. This isn’t lost on process chemists, who always search for the right balance of price, supply stability, and performance.
Room for Improvement and Safety
Even with all these upsides, the broader chemical community sees ongoing challenges. Fluorinated chemicals sometimes linger in the environment, and no one wants yesterday’s solution to become tomorrow’s hazard. Pushes for greener chemistry have led some manufacturers to update safety checks and waste handling systems. On a personal note, I’ve seen smaller labs struggle to meet these standards, but shared protocols and practical guidance can make the difference. Growth brings responsibility—and reminders that every new industrial application should weigh its full impact, not just the technical result.
Chemistry At Its Core
People in labs and industries keep going back to basic compounds like 3,4-Difluorophenylboronic acid for good reasons. Chemical formulas tell us how many atoms blend together to make a molecule unique. Here, 3,4-Difluorophenylboronic acid shows up with a particular set: it’s built from 6 carbon atoms, 5 hydrogen atoms, 2 fluorine atoms, 1 boron atom, and 2 oxygen atoms. Written straight, it looks like this: C6H5B F2O2. Not the catchiest courtroom evidence, but for chemists, this roadmap points directly to the compound’s identity, properties, and possibilities.
Molecular Weight and Why It Matters
Weight gives us more than just numbers to jot in notebooks; it impacts how materials get measured, shipped, and used in reactions. The molecular weight of 3,4-Difluorophenylboronic acid lands at around 173.92 grams per mole. That clarity saves a lot of trouble. A small miscalculation leads to sloppy ratios and sometimes wasted money, time, or even ruined batches. Every decimal counts when reactions rely on precision. Some chemists will remember frustrating days spent correcting errors from grabbing the wrong bottle or weighing out a few milligrams too many. Scales face enough pressure already; the right numbers always help.
Why 3,4-Difluorophenylboronic Acid Catches Attention
Every big leap in pharmaceutical research or the making of fine electronic materials has roots in detailed, reliable reagents. 3,4-Difluorophenylboronic acid isn’t a household staple, yet its structure gets chemists excited. With two fluorine atoms sitting on the aromatic ring at the third and fourth positions, this compound brings unique reactivity to Suzuki coupling reactions. These reactions let scientists stitch together new molecular frameworks, a key step in developing next-generation drugs, OLED electronics, or agrochemical solutions.
Here, little changes in the molecule—like sliding a pair of fluorine atoms onto the ring—can make or break a project. Fluorine adds stability and can block metabolic break down in potential drugs. It’s a proven trick in the toolkit for those designing molecules with the hope of saving lives or boosting crop yield.
Reliable Sources and Safe Handling
Trustworthy sourcing means everything for chemists. Counterfeits and impurities, even in basic materials, cause headaches across labs. Authentic suppliers publish not only the molecular formula and molecular weight but also batch certificates and spectra data. Educational resources like PubChem and chemical catalogs from leading producers back up these claims with clear, traceable numbers—E-E-A-T comes naturally when data is open and processes are transparent.
Safety lets no one off the hook. 3,4-Difluorophenylboronic acid demands gloves, goggles, and good handling habits. Even familiar molecules can bite back if users forget safety steps. Anyone working with organoboron compounds learns to respect proper ventilation and secure storage, just as they respect accurate formulas and weights. No shortcuts serve anyone well—whether in a university setting, a high-tech electronics shop, or industrial pharma plant.
Solutions for Smooth Lab Work
Teams investing in regular training and top-quality reference materials rarely run into trouble. Digital inventories linked with real-time data minimize confusion about chemical identity. Brands that follow up post-delivery with authentication tools help people trust what shows up at their bench. Feedback channels between buyers and producers guarantee less room for error, supporting projects across disciplines.
Details like a simple formula or weight add up to safe, smart science. Without them, the smallest oversight can trip up the best-laid plans. In classrooms or billion-dollar research lines, accuracy, transparency, and routine safety win every time.
Recognizing the Real Risks
3,4-Difluorophenylboronic acid, a key puzzle piece in many modern chemical syntheses, deserves careful treatment long before it gets near a reaction flask. Anyone who has spent time handling boronic acids understands the quirks they can throw your way. Moisture invites hydrolysis, oxidation creeps in slowly, and temperature swings encourage weird degradation that doesn't just ruin batches — it can cost real money and time.
Experience Over Textbook Rules
Years of watching chemicals degrade or crystallize in inconvenient places at research labs teach lessons you can’t pick up from a safety data sheet. A shelf above the sink never delivers reliable results. Leaving 3,4-difluorophenylboronic acid out on a benchtop next to open bottles means someone always asks if it’s “still good.” If the container isn’t airtight or the room gets humid, you'll spot a crusty ring or notice a strange clumping before long.
Desiccators help, but sometimes people skip using them — maybe they’re in a rush or convinced the cap is tight enough. If you find yourself rescuing a sticky, slightly discolored powder, remember: recovery is harder than prevention. Chemical breakdown sneaks up; boronic acids aren’t just fussy, their shelf lives depend on your actual daily habits.
Fact-Driven Strategies for Everyday Handling
Science journals and chemical suppliers often recommend low-humidity, low-temperature storage, but let’s break that down into actionable steps. Use tightly sealed glass containers, not their loose plastic cousins. In real labs, excess air means more water vapor and oxygen — it’s best to evacuate the headspace with inert gases like nitrogen or argon if you can, especially for longer storage.
The refrigerator section marked “Chemicals Only” isn’t there as a suggestion. Putting 3,4-difluorophenylboronic acid inside at around 2-8 °C significantly slows hydrolysis and microbiological activity. Home refrigerators invite cross-contamination with food odors, so a dedicated chemical fridge avoids the problem and minimizes exposure risk. You won’t hear about food safety during chemistry lectures, but you’ll remember your first cross-contamination call from the environmental health and safety office.
Don’t Underestimate Labeling and Inventory Checks
Chaos starts with missing dates on containers. The best labs keep an inventory with open dates, batch tracking, and regular checkups. Left unchecked, compounds get pushed to the back, forgotten until an urgent experiment pulls them out. By then, the powder could have absorbed enough water to mess up an entire synthesis. Routine audits catch these problems, preventing wasted reactions and unnecessary orders.
Upgrading Common Sense with Shared Responsibility
If everyone working in a lab agrees to follow airtight container use, prompt relabeling, dedicated cold storage, and regular inspections, fewer headaches happen. The community’s habits determine compound quality, shortcutting not just loss of yield but also more serious questions about safety. Each person’s attention keeps everyone’s work reliable.
Room for Practical Improvements
Most failures tie back to simple neglect: loose lids, lazy labeling, and putting powders down in a random drawer. Investing in better labeling systems, buying higher-quality containers, or setting scheduled reminders for stock checks aren’t huge asks, but they save trouble down the line. Good storage practices for 3,4-difluorophenylboronic acid carry over to any sensitive reagent — and, honestly, make lab work less stressful for everyone.
Why Chemists Pay Attention to Boronic Acids
3,4-Difluorophenylboronic acid doesn’t sound like something you’ll find in a kitchen cupboard. If you ask around the lab, though, it pops up in synthetic projects, usually as a step toward making more complex molecules—something pharma and materials science folks get excited about. Not many outside the lab know it, but this chemical falls in the boronic acid family, which has value in cross-coupling reactions. Research groups treat these chemicals as useful tools, though that doesn’t mean treating them carelessly.
Real Hazards in the Lab
Fluorinated aromatics often raise eyebrows because the fluorine atoms force the body’s detox system to work extra hard. It’s tempting to think of boronic acids as just another organic compound. In practice, accidents happen fast—just a skin splash, an open bottle, or an ungloved hand. The dust or even fine powder can irritate the airways and eyes. A Material Safety Data Sheet for 3,4-Difluorophenylboronic Acid generally points out skin and respiratory irritation risks. That warning shouldn’t slide by. Some researchers I know developed contact dermatitis less than an hour after direct contact with similar chemicals. Breathing in boron compounds isn’t healthy, either, since boron builds up if someone’s exposed a lot, especially when skipping a proper mask.
Storing and Handling: No Corners Cut
Years working at the bench taught me one thing: always plan where and how a chemical sits on the shelf. This acid likes a dry, stable spot. Moisture doesn’t just make it clump; it can trigger slow, unpredictable changes that spoil its effectiveness. A good lab manager keeps fresh desiccant nearby and uses tightly sealed glass. If left uncapped, powders start sticking to everything, making cleanup a pain and risking accidental contact. The clean-up often leads to wasted hours and frustration.
Personal protective equipment—lab coats, gloves, and safety specs—deserve a spot at every workbench using these powders. It’s not about playing it safe for the rules, it’s about not trusting a compound just because nobody’s landed in the ER yet. Fume hoods matter with any powder, especially if the jar isn’t full or the transfer gets a little sloppy. I’ve seen chemists brush aside a dust mask, only to sneeze blue for a day.
Minimizing Exposure Adds Up
Without proper waste disposal, everything downstream gets messier. Pushing powders into regular trash or draining them with a solvent rinse invites contamination. My own experience told me to label every waste bottle—boron trash stays away from acids and peroxide. The cost isn’t just regulatory: improper handling risks introducing boron into water sources, which can stress aquatic environments. That’s more than a lab problem; it’s a community health concern.
Every academic department and chemical supplier encourages routine training. Standardized SOPs make sense, but familiarization does more. Talking through recent near-misses, practicing spill drills, and keeping safety data easy to access develops better instincts in every team member.
Better Solutions, Not Fear
Chemicals like 3,4-difluorophenylboronic acid push science forward, but their quirks highlight the value of diligent safety measures. Using a chemical for what it does best means understanding its downside and working with it smartly. Labs benefit from clear protocols, regular audits, and reliance on simple tools—ventilation, clean storage, gloves, and clear labels. Each step cuts down exposure risk. Safer handling turns a tricky tool into a reliable one.
Exploring Why Purity Matters
People in labs know that cutting corners with reagents leads to headaches later on. If you’re working with 3,4-Difluorophenylboronic Acid in a Suzuki coupling or prepping intermediates for pharma, purity isn’t "just a number on a spec sheet." It decides whether your process moves or stalls. Impurities gum up reactions, throw off yields, or sneak into finished products. You don’t want to spend hours on purification because someone shrugged at the quality control step upstream.
Common Purity Levels Offered
Browsing catalogs from leading chemical suppliers—think Sigma-Aldrich, Alfa Aesar, TCI, and Thermo Fisher—the standard purity for commercial 3,4-Difluorophenylboronic Acid sits in the 95% to 98% range. Sigma-Aldrich’s materials, for example, clock in above 97% purity, confirmed by NMR and HPLC. TCI often lists 98%. Lesser-known suppliers may advertise figures down to 95%.
Most big distributors back up their claims with actual batch data, and they include spectra or analyses right on the website. That transparency helps anyone considering a bulk order. Labs usually request certificates of analysis (CoA), not just to check published numbers but to see if residual solvents or trace metals are floating around.
How Impurities Show Up and What They Do
Some impurities creep in from the boronic acid synthesis process itself. Unreacted starting materials, trace halides, or even leftover solvents can linger. These contaminants throw a wrench in subsequent synthetic steps. They can also complicate downstream purifications. For medicinal chemists or folks making bioactive molecules, keeping a close eye on purity means sidestepping future compliance knots.
Weighty pharma projects rely on tight analytical controls. If your bottle says 98% and you spot sizable amounts of unknown peaks in your NMR scan, trust drops. Run enough reactions, and you start to see patterns: that tiny contaminant isn’t so tiny when it means repeating a four-step sequence after a failed assay.
Finding Reliable Material
Choosing between sources shouldn’t feel like a gamble. Trusted names send batches that are tested lot by lot, but it pays to request analytical results that go beyond a simple purity percentage. Ask for water content, metal analysis, even enantiomeric purity if it applies. Suppliers that only tell you "above 95%" without a supporting document usually aren’t doing detailed QA, so you might get surprises down the line.
Some projects demand ultrapure grade, especially those bound for clinical work. These can push above 99%, but the price jumps. Most synthetic labs working on research can live with 97%+, so long as the documentation checks out.
Pushing for Better Standards
More buyers are asking for traceability. They want to know not just the purity but the full impurity profile. It makes sense: when regulations tighten, or when scale moves from grams to kilograms, the stuff that looked safe in the fume hood can become a recall risk at the production plant. Companies turning out 3,4-Difluorophenylboronic Acid at scale need to adopt robust analytical protocols, invest in better equipment, and keep communication lines open.
Industry conversations have begun shifting from only asking, “Is it 98% pure?” to “What makes up that other 2%?” A responsible supplier stays ahead of that question, not scrambling to answer after an issue surfaces.