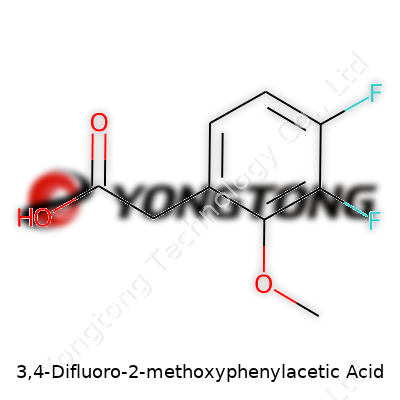
3,4-Difluoro-2-methoxyphenylacetic Acid: A Deep Dive
Historical Development
The story of 3,4-Difluoro-2-methoxyphenylacetic acid goes back to the late twentieth century, a period marked by an uptick in the exploration of fluorinated aromatics within pharmaceutical and agrochemical labs. Chemists noticed pretty early on that dropping a few fluorine atoms into a phenylacetic acid backbone brought out fresh biological properties. It’s the result of researchers looking for better metabolic stability and improved receptor binding—after all, fluorine’s electronegativity often flips the script on reactivity. I’ve watched similar compounds get tweaked and tailored in medicinal chemistry meetings, with each new substituent sparking rounds of speculation about downstream effects. This compound became a subject of in-depth study once chemists connected those small atomic changes to tangible pharmacological outcomes, underlining how advancing chemical knowledge often hinges on taking risks with synthesis and analysis.
Product Overview
3,4-Difluoro-2-methoxyphenylacetic acid sits within a family of building-block molecules for organic synthesis. Its chemical skeleton—an acetic acid chained to a difluorinated, methoxy-substituted benzene ring—sets it up as an intermediate in crafting specialty pharmaceuticals and advanced agrochemical agents. This compound caught the attention of both researchers and manufacturing specialists due to both its reactivity and its ability to serve as a scaffold for branching off into more complex molecules. In practice, it's delivered as a crystalline solid, usually tightly sealed from air and moisture, and each fresh sample carries a data sheet loaded with information about purity, melting point, and handling requirements.
Physical & Chemical Properties
This acid shows up as a white to off-white solid with a moderate melting range, often hovering around 75–80°C. Tossing it onto a balance in a dry room, you’ll notice it doesn’t pull moisture out of thin air like some carboxylic acids. Its moderate solubility in polar organic solvents gives it an edge in reaction setup—think DMF or DMSO for challenging couplings. The two fluorine atoms on the aromatic ring not only suppress metabolic degradation but also modulate the acidity and electron distribution, which can adjust reactivity for downstream chemistry. Its methoxy group contributes a touch of electron donation that sometimes tips the scales during substitution reactions.
Technical Specifications & Labeling
Any supplier serious about quality control slaps a clear-cut label onto the bottle, covering identity confirmation by NMR and mass spectrometry, often with a minimum purity of 98% GC or HPLC. The certificate of analysis spells out limits for water content and residual solvents since even trace moisture can disrupt later stages of synthesis. You see specific lot numbers, batch dates, and storage recommendations: cool, dry, and dark. There’s never any guessing about the material’s chemical identity—every ampoule or drum comes cross-referenced to its matching analytical records, ensuring reliability batch after batch.
Preparation Method
Chemists get to this target molecule using a strategy that leans on classic organic transformation sequences. One common route starts with methylation of a suitable phenol, and the introduction of fluorine can rely on selective halogenation with reagents like Selectfluor or DAST, followed by a side-chain acylation or a homologation approach that drops in the acetic acid unit. Protecting groups, anhydrous solvents, and TLC plates become well-used tools throughout the sequence as side-reactions can eat up time and yield. Yields and purity go up when the route gets tailored to the scale of production, with green chemistry now pushing development teams to minimize hazardous reagents and optimize waste management.
Chemical Reactions & Modifications
The reactive centers stay on the aromatic ring and the acid function. The fluorine atoms tilt aromatic substitution toward nucleophilic mechanisms, so introducing further groups invites careful selection of activating or directing groups. The methoxy substituent helps steer electrophilic aromatic substitution. That carboxylic acid group waits for esterification, amidation, or reduction—transforming into alcohols, amides, or even completely different core scaffolds for drug candidates or crop protection molecules. In the lab, I’ve seen this backbone perform during Suzuki-Miyaura or Heck reactions, linking up with boronates or alkenes to construct bulkier structures.
Synonyms & Product Names
Depending on the catalog or language, this compound goes by names like 2-Methoxy-3,4-difluorophenylacetic acid and DFMPA. In technical documents, it often appears under its systematic IUPAC moniker. You’ll spot it in chemical libraries, screening collections, or internal company databases with those alternate labels, so reading cross-references on safety data sheets avoids mix-ups in busy lab environments.
Safety & Operational Standards
Lab teams treat 3,4-Difluoro-2-methoxyphenylacetic acid with respect, following the same golden rules drilled in by decades of chemical safety culture. Personal protective equipment (PPE) stays on: gloves, lab coat, and splash-proof goggles, all day long. Chemical fume hoods keep breathing air clean, especially since phenylacetic acids—fluorinated or not—raise concerns about skin sensitization and respiratory irritation. Spill containment and waste disposal track local and federal environmental rules. If an emailed MSDS warns of possible organ toxicity, nobody bends those guidelines. All containers get labeled in plain language, and any transfer between storage and work spaces includes secondary containment.
Application Area
This compound plays its part in medicinal chemistry, agrochemical development, and materials science. I remember a time a research team used it as a key intermediate on the path to a novel class of non-steroidal anti-inflammatory drugs. Its core structure gives pharmaceutical chemists leeway in attaching side chains or swapping out substituents to chase improved activity or lower toxicity. Crop protection scientists lean on fluorinated aromatics like this one to chase enhanced pest resistance in plant treatments. In some corners, it turns up in the preparation of imaging agents or specialty polymers, taking advantage of the altered electronics and metabolic pathways that fluorine brings.
Research & Development
It’s a favorite in research labs pushing the boundaries of drug metabolism, thanks to its sturdy C–F bonds and its ability to slip into larger molecules with minimal metabolic penalty. Fluorinated phenylacetics get checked for new biological roles using high-throughput screens. Computational chemists run docking models to see how substitutions impact target binding, guiding the synthesis teams’ next moves. Open-access databases point to dozens of research articles every year mapping routes for scale-up and green chemistry improvements, each project building on lessons from before.
Toxicity Research
Toxicity profiling for this compound matters a lot before it can jump into commercial processes. Fluorinated aromatics don’t always play by older rules—both acute and chronic exposure can stir up surprises in cell culture and animal models. Some results show that metabolic breakdown doesn’t happen as fast, which can be a blessing or a headache depending on where the compound ends up. Industry partners run detailed ADME (absorption, distribution, metabolism, excretion) pipelines, looking for signals in liver enzyme assays, mutagenicity screens, and off-target binding to avoid unwanted consequences down the road.
Future Prospects
Looking ahead, 3,4-Difluoro-2-methoxyphenylacetic acid holds promise as a springboard for more selective pharmaceuticals and durable agrochemicals. Synthetic chemists will keep searching for cleaner, safer ways to build this molecule on bigger scales, while regulatory agencies scrutinize its toxicity footprint. Advancements in automated chemistry and reaction engineering might drop costs and improve yields. I can see a push toward designing greener fluorination methods, easing environmental pressures without losing any chemical utility. The next generation of medicinal chemists and process engineers will likely find new pathways to put this molecule’s unique skeleton to use, driven by fresh ideas, regulatory restrictions, and the relentless need for innovation in health and agriculture.
Why Purity Is a Touchstone in Chemical Research
Nobody in the lab has ever been thrilled by the phrase “unknown contaminants.” Working with 3,4-Difluoro-2-methoxyphenylacetic acid, you count on a certain level of transparency about purity. This isn’t just about keeping beakers clean or checking another box on a procurement form—reproducibility and reliability live or die by the percentage stamped on that certificate of analysis. A metric like 98% purity means confidence in the reaction outcomes, consistent spectral values, and—most important—interpretation of any data downstream.
Why Purity Levels Matter Beyond the Obvious
A batch with 98% purity shaves off unwanted variables during analysis. Take chromatography. Even a small trace of impurity can show up as a ghost peak, muddying results and, worse, creating headaches during scale-up. I remember working on an aromatic acid where the supplier listed “not less than 97%.” Every time I had to double-check my HPLC—was that tiny peak the impurity, or am I dealing with a fresh decomposition? That’s time lost, resources wasted, and doubt cast on the quality of follow-on products.
Impact on End Users and Broader Supply Chains
Anybody pushing molecules toward pharma development can’t gamble on uncertain quality. Only a hair-thin margin separates a useful intermediate from a safety risk, especially during late-stage synthesis. Regulations don’t just come from the bench—they’re watchdog protocols for every element along the pipeline. Companies offering 3,4-Difluoro-2-methoxyphenylacetic acid at 98% or higher serve more than the end users; they support safety and integrity up and down the chain. The expectation gets magnified when that compound lands anywhere near clinical research.
Verification and Transparency: No Shortcut
If the certificate of analysis provides only a cursory “min. 98% chemical purity,” you need backup—NMR spectra, GC-MS traces, or HPLC data. Rigorous QC doesn’t just exist to keep procurement teams happy—it gives researchers evidence they can trust. Anything less just fuels uncertainty and extra spending on in-house testing. I once received a sample labeled as “98% pure,” but the batch came out at 95% when I checked with my own instruments. That three percent was enough to change the whole course of our experiment.
Solutions in Maintaining High Purity
Sourcing matters as much as science. Sticking with suppliers who operate GMP-certified or ISO-accredited facilities puts risk in check. Requesting third-party purity certificates or independently verifying samples before full-scale projects saves headaches later. Discussing expectations—requesting lot-specific documentation or recent spectral data—helps avoid trouble for everyone. Finally, storing and handling chemicals according to recommendations ensures purity doesn't drift before you even break the seal.
Trust Built on Numbers and Experience
Purity claims are more than sales copy. Each decimal matters. In my own projects, the difference between problematic variability and smooth progress has often come down to a fraction of a percent— nothing abstract about that. For 3,4-Difluoro-2-methoxyphenylacetic acid, asking the right questions and demanding real, testable answers isn't just about compliance. It’s how labs, teams, and—ultimately—fields of research move forward with clarity.
Storing Chemicals with Care
Most folks working in a lab understand the worry that comes with handling specialty compounds. 3,4-Difluoro-2-methoxyphenylacetic acid isn’t the world’s most famous chemical, but you’ll find it popping up in organic synthesis, especially within medicinal chemistry circles. When daily routines revolve around safeguarding sample integrity, proper storage quickly becomes a bedrock principle—not just a line in the safety manual.
Temperature Means More Than Comfort
Anyone who’s lost a sample to a careless temperature swing knows the pain of wasted effort and budget. 3,4-Difluoro-2-methoxyphenylacetic acid prefers cooler environments. Refrigerators at 2–8°C keep the compound stable, slowing unwanted degradation reactions such as hydrolysis or oxidation. High temperatures accelerate chemical breakdown, spurring impurity formation and changing physical properties. Even a few days above recommended temperatures can lead to murky data or, worse, a dangerous shift in chemical behavior.
The Importance of a Dry Setting
Humidity might seem like a minor annoyance to many, but even a slight uptick in moisture spells trouble for acid derivatives. Those working in humid regions quickly learn the value of sealing compounds tightly and avoiding any open-air exposure. Desiccators, equipped with silica gel or other drying agents, provide an added layer of protection against ambient water vapor sneaking into containers. A careless habit of leaving caps loose or bottles open shortens shelf life, sometimes invisibly swirling trouble into your next reaction without warning.
Container Choices Matter
Some might reach for plastic vials out of habit, but sensitive acids—especially when discussing aromatic fluorinated variants—favor glass. Polyethylene and polypropylene containers may leach traces or allow vapors to seep through over time. Amber glass adds another level of defense, minimizing any photo-induced reactions that otherwise degrade your compound. Each time a researcher swaps suitable containers for a quick fix, risks sneak in and chip away at reliability.
Sensible Labeling and Inventory
Working with years-old samples from unmarked bottles never leads to good results. Adherence to labeling serves more than just administrative policy. It ensures anyone picking up that bottle—whether graduate students or experienced chemists—knows what’s inside, its added date, and any special hazards identified by the supplier. Too many accidents in the lab start with assumptions about what’s in a given container. Serious research doesn’t thrive on guesswork.
Personal Experience in the Trenches
Years spent in both academic and industrial labs taught me that well-documented storage protocols mean fewer headaches down the road. I recall repeated cases where improper temperature or missing labels led to botched experiments, frustrated project teams, and expensive reordering. It rarely takes a disaster to appreciate good storage habits—just a few trips back to square one.
Practical Next Steps
Other than keeping acids in a fridge, I’ve always set reminders for periodic sample checks. Regularly inspecting for discoloration, unusual odors, or container leaks often catches early warning signs. Combining careful storage with staff training—reinforcing the importance of sealing, labeling, and monitoring—makes all the difference. Chemicals may gather dust on the shelf, but vigilance ensures safe, effective research and preserves the hard-won results researchers chase.
Trust Is Earned With Transparency
Ask someone in the food or supplement industry about certificates of analysis, and you might get a tired look. Folks like me who’ve worked with suppliers and seen products change hands ten times before they end up in a bottle—well, we care about that one piece of paper more than any shiny label. A certificate of analysis, or COA, backs up claims about purity, potency, and safety. Without it, you’re tossing a lot of trust out the window and hoping nothing comes back to bite you.
Consumers Demand Proof
People don’t take a company’s word for it anymore. Scandals around contaminated pet food, mislabeled supplements, and fake olive oil drove home a lesson: proof is better than promises. In my own kitchen, supplements with QR codes or batch-specific COAs feel safer than the ones that avoid the subject. I know what I’m paying for. Lab results with batch numbers and clear limits for common contaminants, heavy metals, or microbial load reflect a company’s accountability to its customers.
Regulators Don’t Mess Around
In the US, the Food and Drug Administration demands accurate information for imported food, supplements, chemicals, and pharmaceuticals. GMP (Good Manufacturing Practice) guidelines outline strict recordkeeping. If you’re found selling anything from applesauce to amino acids without supporting documentation, fines and recalls follow. Europe and many Asian countries enforce their own rules—sometimes even tougher. Missing COAs often grind imports to a halt at port and cost both time and money.
Quality Control Can’t Happen Without Data
I’ve done the quality game on the buying side. You spot issues with odor, texture, or color—those you can test yourself. But chemical identity, allergens, solvent residues, or pesticide levels? That calls for outside help. A real COA lists the testing method, who performed it, and the results for that exact batch. This makes all the difference whether you’re baking gluten-free cookies or manufacturing a protein shake. It’s the difference between guessing and knowing.
How Companies Can Step Up
Many brands now link COAs to each batch online, giving customers a quick and clear look. Others keep them stashed away, handing them out by request. Open access isn’t just about compliance—it’s about respect for the people who buy the product. Companies gain trust by keeping records organized, choosing ISO-certified labs, and training their teams to spot fakes.
Retailers can pressure suppliers to provide COAs before products hit shelves. This protects them and signals to customers that corners are not being cut. Labs, on their end, push for more precise, transparent, and error-checked reporting.
Final Thoughts
People deserve to know what’s in what they buy. COAs aren’t just paperwork—they’re roadmaps guiding better decisions, signaling trust, and laying the groundwork for long-term safety. Expecting a COA isn’t just picky; it’s smart shopping, and it keeps everyone honest.
The Reality of Sizing Choices
Working in a chemistry lab, you quickly notice how much time and energy get lost chasing the right reagent packaging. Walk into storerooms, and you see powder, crystals, and liquids bottled in everything from a few grams to hefty kilos. For 3,4-Difluoro-2-methoxyphenylacetic Acid—an intermediate that pops up in pharmaceutical and agrochemical research—packaging isn’t just about a glass or plastic container. It shapes workflow, cost control, and even lab safety.
The Typical Range: Small to Large
Producers of this compound usually offer several standard sizes: 1 gram, 5 grams, 10 grams, and 25 grams for R&D and specialty synthesis. Step up to pilot plants or small-scale manufacturing, and packaging jumps to 100 grams or 250 grams, sometimes stretching to a kilo or more. Once or twice, I’ve seen requests for custom sizes, especially when collaboration means tighter project budgets and strict timelines.
A scientist ordering this compound in 1-gram bottles usually runs screening assays, not kilo-scale syntheses. It’s a meaningful detail, because with high-purity chemicals, shelf-life and the risk of contamination eat up resources if you work with bigger-than-needed packages. Overbuying leaves you with costly leftovers. Underbuying derails experiments midway. Getting that balance right pays off in time and money.
Market Drivers Behind Packaging Decisions
Suppliers settle on these packaging options from years tracking customer habits. Pharmaceutical companies need flexibility. Some projects only reach for milligrams, but scale-up can mean kilograms. Smaller academic labs stretch funding with smallest-size bottles, but contract manufacturers often require 250 grams, 500 grams, or even custom drum sizes for pilot trials.
Considerations go beyond just volume or mass. The packaging material itself matters because phenylacetic acid derivatives sometimes absorb moisture or degrade under light. That means amber glass is standard, with tamper-proof seals. Last batch I ordered came double-sealed, labeled with batch numbers and expiry dates—details that keep labs compliant during audits.
The Importance of Smart Packaging Choices
I’ve learned the hard way that inconvenient packaging slows down projects. Opening a 100-gram jar every week for tiny scoops encourages mistakes and reduces purity. In contrast, manufacturers that offer a well-thought-out size range let researchers pick exactly what’s needed, avoiding spoilage and loss.
By working with suppliers who listen to real feedback, labs can focus budgets where they matter, boost productivity, and keep up with evolving research needs. Some suppliers now pair size options with detailed safety sheets and handling guidelines, making it easier for newer lab staff to minimize errors.
Practical Solutions for Labs and Suppliers
Instead of just accepting what’s on a catalog, lab managers and chemists can ask for packaging that fits their workflow. Group orders with allied labs, or talk with vendors about custom sizes if regular options miss the mark. Vendors that actually communicate with end-users stay ahead, while those relying on a one-size-fits-all model create unnecessary friction.
Transparent batch records, traceable barcodes, and clear labeling aren’t just regulatory boxes to tick—they make inventory tracking much more reliable. With careful attention to storage, handling, and disposal, the right packaging sizes turn from afterthoughts into productivity drivers and cost-cutters for everyone in the supply chain.
Waiting for Your Order: Why Timing Matters
Putting in an order and then counting down the days is something everyone has dealt with. Whether it’s a new phone, furniture, or parts for your business, delivery isn’t just about convenience. I remember waiting for a specialized laptop during a crunch period at work. Every delay meant scrambling to meet deadlines. Delays ripple out—plans get pushed, commitments fall through, and sometimes even money is left on the table.
How Lead Time Shapes Business and Daily Life
Lead times stretch far beyond the shipping confirmation email. In my own consulting years, I saw supply chains break down because one shipment landed a week late. Busy seasons hit, demand spikes, and suddenly your supplier needs extra days or weeks. A trucking hitch, customs slowdown, or missing paperwork can turn an expected delivery window into a guessing game.
Long lead times can handicap a small local business. A bakery that needs packaging might miss a big holiday rush, and a builder waiting for parts can lose contracts to someone who delivers faster. On the personal side, late deliveries affect everything from birthday gifts to home renovation schedules.
What Shapes Delivery Timing?
Many variables affect how long an order takes to arrive. If the product comes from overseas, customs and international shipping each add their own delay. Local inventory also plays a key role. When stock sits in a nearby warehouse, you might get what you ordered in a day. Restock cycles, backorders, and rare colors or models can add days or weeks. My friend once waited two months for a red sofa, just because the factory made fewer of that color.
Retailers do their best to give honest estimates, though things don’t always go to plan. Weather, staff shortages, and even strikes can grind shipments to a halt. Sometimes, one missing part keeps an entire order from shipping. Experience taught me never to trust online estimates completely during busy times like holidays or major sales events.
Building Trust Through Transparency
People value clear, honest communication. If a company sets reasonable expectations and keeps buyers in the loop, the waiting game gets easier. It goes a long way when you get updates—good or bad—without having to chase down customer service. Just last year, I ordered home gym equipment that hit a customs snag. The company updated delivery dates and explained the holdup. No surprises, no frustration.
What Can Make Delivery Faster?
Better forecasting helps companies keep items in stock. AI and data analytics show promise in predicting demand spikes. Simple improvements, like letting customers know about stock shortages before checkout, make a world of difference. Some businesses now give tiered delivery options. Paying extra for rush delivery makes sense if needed, but clear standard timeframes matter just as much.
Even the most reliable system can stumble, though. Relying on one supplier or shipper leaves everyone exposed to breakdowns. Building in buffers and working with backup partners can soften the blow of a late arrival. From a customer’s view, the ability to track orders in real time creates trust—and keeps the anxiety at bay.
Final Thoughts on Lead Times
Waiting for an order never feels easy. Managing expectations with clear communication and realistic estimates turns an uncertain process into something people can depend on. In the end, lead times shape more than deliveries—they shape our plans, our work, and even our trust in a brand.