3,4,5-Trifluorophenylboronic Acid: A Look Beyond the Beaker
Historical Development
The journey of 3,4,5-Trifluorophenylboronic Acid (CAS 138564-59-7) reflects the rich evolution of organoboron chemistry. In the late twentieth century, researchers started probing boronic acids for their potential in Suzuki-Miyaura cross-coupling. Hampered by the limits of older halogenated compounds, chemists turned toward more versatile building blocks. Through persistent effort, they unlocked methods to assemble aryl boronic acids sprinkled with fluorine atoms, fueling both academic and industrial labs. I saw first-hand during graduate work how access to these trifluoroaryl compounds expanded synthetic possibilities, particularly in pharmaceutical research chasing improved metabolic stability and bioavailability.
Product Overview
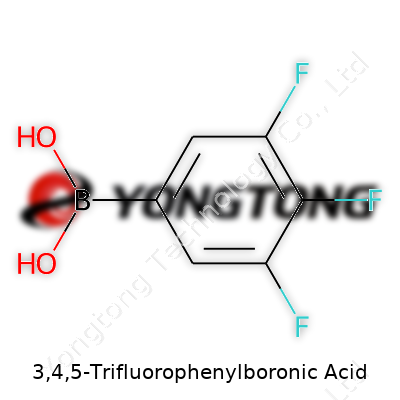
3,4,5-Trifluorophenylboronic Acid belongs to the family of boronic acids, standing out with its triad of fluorine substituents along the aromatic ring. As a white to off-white powder, its appearance hints at the robust chemical resistance gained from fluorination. High purity chemicals like this support the foundation of cross-coupling reactions which build complex molecules for agrochemicals, electronic materials, and drug candidates. Having handled it in the lab, I found its preparation consistent and its quality predictable — two things research teams need to keep projects moving.
Physical & Chemical Properties
This molecule's structure gives it a melting point ranging from 148 to 152 °C, a trait useful for confirming identity. Solubility tells another story — it dissolves well in polar organic solvents like DMSO and methanol, which matters when designing new synthetic routes. The compound resists water, thanks to the stubborn nature of fluorine, but its boronic acid group stays reactive. In the flask, these features guide every step, from purification to handling.
Technical Specifications & Labeling
Bottles arrive typically labeled with molecular formula C6H2BF3O2 and formula weight 192.89 g/mol. Quality controls check for purity above 98% by HPLC. Some suppliers boost safety by indicating hygroscopicity — moisture transforms the acid, impacting reactivity. Storage advice pushes for a cool, dry place, tightly sealed from air and water. Details on batch number, lot, and shelf life empower traceability, crucial both for scientists and regulatory compliance.
Preparation Method
Lab synthesis kicks off with a trifluorinated aryl precursor, usually through halogenation of phenol derivatives. The route typically employs lithiation followed by reaction with trialkyl borate, then acid workup. I have watched researchers optimize each step, trimming costs by recycling solvents and controlling temperature during lithiation. Purity demands careful filtration and crystallization, with routine NMR used to confirm success. The reproducibility of these protocols keeps supply chains reliable for chemists worldwide.
Chemical Reactions & Modifications
Thanks to its boronic acid group, this building block transforms quickly under palladium-catalyzed Suzuki-Miyaura reaction conditions. Whether forming biaryl linkages or more exotic architectures, each reaction brings opportunities for new medicinal agents or advanced materials. Modifying the substituents or converting the boronic acid to boronate esters can adjust solubility or reactivity for specific applications. The trifluorinated ring increases the molecule's resonance stability, while those fluorines help tune the electron density, influencing downstream reactions in ways I have seen yield sharper selectivity and more potent biological properties.
Synonyms & Product Names
Researchers recognize this molecule by names such as 3,4,5-Trifluorobenzeneboronic acid, Trifluorophenylboronic acid, or the systematic (3,4,5-trifluorophenyl)boronic acid. Many laboratory catalogs add acronyms or supplier codes, but the CAS number remains the reference point for precise ordering. Over years, I have seen synonyms create confusion, especially across supplier catalogs, so double-checking identifiers saves time and trouble.
Safety & Operational Standards
Safety data call for gloves and eye protection, as boronic acids can irritate skin and mucous membranes. The material does not burn easily but releases hazardous fumes if overheated. Engineers stress good ventilation and discourage open containers in humid conditions because boronic acids, even ones as robust as this, soak up water quickly. Disposal through high-temperature incineration prevents environmental buildup, a practice set by tough federal and EU standards. During my work with fluorinated aromatics, spill kits and protocols for quick cleanup were essentials, not afterthoughts — protecting both people and the environment.
Application Area
For synthetic chemists, 3,4,5-Trifluorophenylboronic Acid represents a trusted partner for building complex molecular frameworks. Medicinal chemistry teams target its use to optimize pharmaceuticals, especially where fluorine's tweaks to metabolic stability or binding selectivity matter for drug candidates. In electronic materials, the more unusual electron withdrawing pattern supports specialty polymers or organic semiconductors. Agriculture embraces derivatives that add pest resistance or longer soil stability to agrochemical formulations. My colleagues in pharmaceutical R&D have relied on this molecule for structure-activity studies, often running parallel syntheses to find the format with the best potency or absorption.
Research & Development
Ongoing research continues to uncover new methods of coupling, faster reaction kinetics, and greener solvents. As digital tools such as AI modeling speed up molecular design, access to reliable boronic acids leans into rapid iteration and scale-up. At conferences, I hear excitement over automated platforms that accept off-the-shelf building blocks like this one — these trends drive demand for ultra-pure, well-documented reagents. Innovation in purification technology and recovery from reaction mixtures pushes green chemistry forward each year.
Toxicity Research
Animal studies show a low acute toxicity profile for boronic acids but caution rises with chronic exposure or accidental ingestion. Fluorinated aromatics pass through the environment less predictably, spurring deeper regulatory scrutiny. Toxicologists weigh the long-term impact in both aquatic and terrestrial food chains, pushing chemists to predict environmental fate and to engineer safer or more biodegradable analogs when possible. My early work analyzing metabolite profiles showed how small fluorinated molecules can bioaccumulate, teaching me to always question persistence and to account for not just immediate toxicity but possible downstream effects.
Future Prospects
The importance of 3,4,5-Trifluorophenylboronic Acid looks unlikely to fade as research pursues molecules with enhanced precision in biological and material applications. Coupling efficiency, green chemistry, and digital inventory tracking shape tomorrow’s supply chain. Several R&D projects aim to recycle boronic acids from waste streams, as sustainability edges higher in procurement conversations. As new fluoroaryl structures find targets in next-generation drugs, sensors, and battery components, reliable sources and self-improving synthetic methods will keep this compound in regular demand. Collaboration between academic groups, industry, and regulators will push both discovery and stewardship forward, promising further surprises and safer, more effective uses.
Everyday Chemistry Gets a Boost
3,4,5-Trifluorophenylboronic acid sounds like a tongue-twister, but it earns real respect in labs and factories. Fluorinated building blocks push the boundaries of new drug candidates and novel materials. I’ve watched bench chemists build better molecules with tools like this—there’s always excitement when you find a “magic” piece that connects things in fresh ways.
Pharmaceuticals Grow Stronger
Modern drug discovery leans into customized molecules packed with functionality. These boronic acids serve as “linkers” in Suzuki-Miyaura coupling, which feels like playing with high-tech LEGO blocks for grown-ups. Numbers from patent filings back up this point: over a third of new small-molecule treatments coming out in the past decade use some form of fluorine setup. 3,4,5-Trifluorophenylboronic acid adds three spots for fluorine at once, letting medicinal chemists tweak activity, selectivity, and even the way the drug moves in your body. This helps design cancer-fighting molecules with fewer off-target effects or ones that stick around longer where they’re needed.
Materials Science Pushes Ahead
Hard plastics, specialty coatings, OLED displays, and new polymers rely on clever molecules. Researchers turn to trifluorinated boronic acids to introduce just the right electronic or physical traits. Watching the film industries chase brighter, more reliable OLED displays, I noticed a sharp jump in using these building blocks. They amplify stability, making materials last against sunlight or chemical exposure, and lend unique effects for light emission and conductivity. These advantages ripple through to the final product. It’s not just the chemistry that counts—manufacturers save costs by reducing degradation and prolonging shelf-life.
Diagnostics and Sensing
Lab tests and point-of-care devices use sensors to pick up small biological clues, such as sugar levels in the bloodstream or traces of disease markers. Boronic acids, including this one, famously bind with sugars and some amino acids. If you’ve seen rapid advances in diabetes monitoring or “laboratory on a chip” solutions, there’s a good chance these components are hard at work behind the scenes. The fluorinated structure helps resist interference from other chemicals and boosts detection reliability. In my professional circle, folks have used boronic-acid sensors for both routine checks and rare diseases, celebrating the speed and accuracy jump over older diagnostic methods.
Tackling the Challenges
Scaling up from the lab to large-scale production often brings worries about cost, safety, and waste. 3,4,5-Trifluorophenylboronic acid isn’t always cheap. Research labs sometimes hunt for alternatives or process tweaks—greener reagents or recycling steps—to keep expenses and environmental impact down. Over the years, process chemists have reported big leaps forward: safer coatings that release less byproduct, ways to rebound and reuse some spent materials, or strategies to make the boronic acid less hazardous during transport.
Looking Forward
Progress in the sciences always circles back to the tools people use. 3,4,5-Trifluorophenylboronic acid shows up across industries because it works so well in precise hands—from crafting sharper medicines to advancing electronics and quickening diagnostics. There’s always room for improvement, particularly around cost and broader access, but each new application tells me researchers and manufacturers value versatility and innovation over buzzwords and short-term gains. Seeing these patterns repeat year after year gives me confidence that this molecule hasn’t finished making news yet.
Unlocking the Chemical Build of 3,4,5-Trifluorophenylboronic Acid
Stepping into the world of organic chemistry, every researcher knows the thrill of tracing a molecule’s intricate connections. In pharmaceuticals, materials science, and agrochemicals, 3,4,5-Trifluorophenylboronic acid shows up as a specialist reagent. No complicated jargon or smoke and mirrors—just a benzene ring carrying three fluorine atoms, all neatly lined up, and a boronic acid group. Its structure doesn’t get much simpler or more useful.
Drawing the Skeleton: What Does the Structure Look Like?
Take a benzene ring. Picture positions 3, 4, and 5 swapped with fluorine atoms. On position 1, attach a boronic acid group—written as B(OH)2. It’s not magic, only careful arrangement. This leaves two hydrogen atoms that haven’t bowed out to their more electronegative rivals. This arrangement isn’t arbitrary. Fluorine atoms pull electrons and shuffle electron density, making the molecule a key player in Suzuki coupling reactions, a bread-and-butter reaction for building complex organics in labs and industry.
Molecular Formula and Geometry
Now, onto the formula: C6H3BF3O2. Six carbon atoms, three hydrogens, a boron, three fluorines, and two oxygens. This count isn’t just bookkeeping. When chemists source or manufacture chemicals, accuracy ensures safety, regulatory compliance, and cost management. Any mistake will ripple through the supply chain and show up right at the bottom line or, worse, on the workbench with failed reactions or hazardous surprises.
Why All the Fuss Over a Boring Ring?
Some might see a heap of fluorines and a boronic acid as just another tweak on a familiar template. That’s missing the forest for the trees. Chemistry is about controlling reactivity. In this molecule, the trifluorophenyl group changes how the compound behaves in water, in organic solvents, or when it bumps into metal catalysts. Fluorine squeezes the electron cloud. That’s why cross-coupling partners—such as aryl halides—find new life attached to the boronic acid. Drug makers lean on such molecules as building blocks for once-impossible compounds.
Real-World Relevance in Labs and Industry
I’ve handled similar boronic acids in real labs, using them to stitch together more complex pharmaceuticals. Clean reactions depend on understanding the quirks of each building block. Misjudging the effect of those three fluorines invites headaches down the line. Think lower yields or odd byproducts. Careful structure analysis and a solid grasp on molecular geometry smooth the way for scalability and reproducibility. This directly supports quality, which major regulators like the FDA or EMA demand for every batch of active pharmaceutical ingredient.
Moving Forward With Safety and Transparency
The handling of 3,4,5-Trifluorophenylboronic acid, like every strong reagent, demands respect. Reliable safety data sheets, up-to-date databases, and transparent sourcing help keep chemists safe. Institutions such as PubChem and ChemSpider hold up-to-date public records of structure, hazards, and regulatory guidelines. Open communication and careful documentation take chemicals from the drawing board to the production line without unnecessary risks or confusion.
Building Solutions for Modern Chemistry
Accurate structural diagrams, fast digital access to molecular data, and trustworthy suppliers build a foundation for safe, scalable, responsible chemistry. Companies that invest in education, share real spectra, and publish reliable batch data make it easier for everyone to meet both innovation and safety targets. Addressing global challenges relies on this level of transparency and precision. That’s worth every bit of attention to a trifluorinated benzene ring dressed in boronic acid.
Real-World Chemistry Demands Respect for Hazards
Dealing with chemicals like 3,4,5-Trifluorophenylboronic Acid takes more than a clean lab coat and tidy workbench. Anyone who spends time working with organic reagents knows that common-sense habits go a long way. Sadly, injuries often come from forgetting the basics—so let’s get those nailed down.
Respecting Storage Requirements: Keep Moisture Out
This compound doesn’t play well with moisture in the air. Over the years, chemists have pulled open careless containers, only to find a crust of ruined reagent. Boronic acids absorb water and eventually break down—money wasted, results ruined. Keep it tightly sealed, and never store it out in the open. Use a desiccator or a dry cabinet. Store the jar at room temperature, but never let sunlight beam down on it. The compound lasts much longer in a cool, dark spot.
Planning for Spills: Gloves Are Not Optional
No one loves wearing gloves for hours. Here’s the reality: this colorless to pale yellow powder may not look dangerous, yet it can irritate skin and eyes. Strong odors don’t tip you off to exposure. Wear nitrile gloves that fit snugly. Don those safety goggles; in a moment of haste, a puff of reagent can end up in your eye. Covering up with a lab coat prevents hands and arms from getting an unexpected dusting. Proper clothes beat a trip to the campus nurse or an expensive call to poison control.
Safe Handling Prevents Future Incidents
Most mishaps happen while weighing out powder. Pouring directly from a big bottle almost guarantees a mess. Use a spatula—preferably one you can clean afterward. Place a sheet of clean paper under your balance and transfer slowly. If any powder spills nearby, clean it up right away. Avoid inhaling dust, so work in a fume hood every time. After finishing, wipe down surfaces and wash your hands with soap, not just a quick rinse with water.
Proper Waste Disposal Matters
Lab work always produces leftover reagent and contaminated glassware. Tossing boronic acids down the drain leads to headaches with environmental health. Gather up contaminated materials—gloves, paper, and old samples—and seal everything in a marked container. Follow your lab’s waste protocols—most places treat boronic acid residues as hazardous waste.
Training Beats Guesswork
Rely on a solid workplace culture that values safety conversations. New students—or anyone unfamiliar with organic reagents—should get hands-on training from more experienced chemists. Everyone in the lab has made small mistakes, but open communication shortens the learning curve and curbs dangerous slips. Safety data sheets belong within reach of the bench; check those before opening any container the first time.
Invest in the Right Storage Supplies
Clear labeling is not decoration. Write the full name, date received, and your name on every container. Airtight glass or sturdy plastic does the job, but check that the cap fits. Never trust a bottle with a warped cap or cracked body. Regular checks on chemical stocks catch storage problems before they snowball.
Final Word: Good Habits Outlast Rules
Safe handling of 3,4,5-Trifluorophenylboronic Acid rests on routines, not regulation. Preserve your chemicals, your data, and your health. Basic respect for these compounds keeps everyone working on their goals, instead of untangling preventable emergencies.
Understanding Purity for a Critical Building Block
3,4,5-Trifluorophenylboronic acid shows up in organic synthesis labs just about everywhere. Its role as a piece in Suzuki coupling keeps it on a long shopping list for anyone working on pharmaceutical or agrochemical projects. Perhaps the most important box to check on any bottle is purity. A quick glance through major chemical supplier websites says a lot: purity for this reagent usually sits at 97% and above. That isn’t trivial. Even the smallest contaminants can wreck a reaction, especially when precision matters or yields are tight. In my own time running reactions, a bottle marked 97% always brought more confidence. For particularly sensitive experiments, vendors sometimes offer even higher-purity, such as 98% or HPLC-purity, though most routine synthesis happens with the typical 97% grade.
Why is such a high standard the rule here? Boronic acids don’t always bring comfort in storage — they can degrade, pick up moisture, or react with the air. Whenever a reaction demands consistency, nobody wants to guess how many impurities will tag along. There’s a direct price-to-performance trade-off: the cleaner the compound, the fewer surprises in the fume hood. Buying higher-purity stocks means fewer headaches during column chromatography and saves researchers those extra steps of cleanup.
Packing Size: From Discovery to Production
Not everyone needs a drum of this compound. Small research teams and university groups usually order in the gram range. One of the largest suppliers lists 250 mg and 1 g vials alongside their 5 g and 25 g bottles. This makes sense for early-stage work or if someone tests a new route that could fail. I remember those days of scraping every last bit of powder out of a tiny glass bottle, hoping it would cover a synthesis run. Big batches run by pharma process groups go larger, with suppliers able to offer 100 g to 500 g bottles or custom bulk packaging if needed. The idea here is flexibility — taking as much as the job demands, no more and no less. For routes close to scaling up, it helps that some suppliers will coordinate custom packaging, keeping supply risks low and waste to a minimum.
Meeting Lab Needs and Staying Safe
This acid isn’t benign. It carries typical risks common to boronic acids: irritation to skin and eyes, and the usual chemical safety precautions — gloves, eye protection, and a source of good ventilation. Proper packaging matters more than most people think. Those small glass vials seem delicate, but robust screw caps and moisture-proof seals keep the acid stable in storage racks. Labs storing larger bottles know to avoid repeated opening and closing, since moisture and air can spoil a whole batch. My old PI kept a personal stash of desiccant packets for every freshly opened bottle, just to keep integrity as long as possible.
Disposal and handling create another set of concerns. Many university shops provide clear protocols, but working with a trusted supplier ensures safety data and SDS sheets come with every shipment. This paperwork helps meet regulations and protects everyone in the lab, especially less experienced students jumping into synthesis for the first time.
Looking Forward: Trust, Transparency, and Sustainable Sourcing
Sourcing materials like 3,4,5-trifluorophenylboronic acid puts a heavy load on trust. Reliable suppliers state what’s in the bottle, provide batch-specific documents, and respond quickly if purity ever falls below standard. Supply chain hiccups in recent years point toward building more partnerships and keeping options open. Some labs, tight on budget, look for long-term contracts or multi-use quantities — keeping research running steady, not wasting money on lost or spoiled materials.
Innovation also points toward sustainable production. Green chemistry initiatives and better waste strategies offer a way to preserve both researcher health and planet health. As this acid keeps its place in advanced synthesis, purity and packaging will remain front-and-center issues for years to come.
What Science Tells Us About This Chemical
People in chemistry labs know 3,4,5-Trifluorophenylboronic acid for its value in making pharmaceuticals and advanced materials. This boronic acid, with three fluorines on the aromatic ring, doesn’t look alarming at first glance—so it might be easy for someone to assume it’s harmless. That belief doesn’t fit with reality on the lab bench.
Hazards Worth Attention
Working around organoboron chemicals always carries some risk. For most, skin and eye irritation ranks high among the concerns. 3,4,5-Trifluorophenylboronic acid can cause a burning feeling if it lands on your hands or splashes in your eyes. Inhaling dust triggers sneezing, coughing, or even shortness of breath for some people. Chronic exposure adds another layer of worry. Extended contact sometimes leads to dermatitis or other skin reactions.
Fluorinated compounds have another side. The trifluoromethyl group makes molecules more persistent in the environment and sometimes trickier for the human body to process. That persistence raises questions for anyone thinking about green lab practices or long-term waste handling.
Experience With Handling
Anyone who spends enough time working with powdered chemicals eventually runs into spills or unexpected dust. One careless scoop can fill the air with fine particles. From personal experience, these powders like to travel—settling on keyboards, desktops, or even the rim of your coffee mug if you’re not careful. That contamination risk means never skipping gloves and always double-checking that safety goggles fit right.
Some solvents amplify the dangers. If the boronic acid meets alcohol or strong base, new compounds can form—sometimes with unpredictable toxicity. That mix calls for using a fume hood every time, keeping incompatible bottles far apart, and making sure all containers clearly state their contents.
Waste Isn’t Just an Afterthought
It’s all too easy to let cleanup slide, but boronic waste deserves respect. My lab learned this lesson when a careless rinse sent residues into the regular drain, leading to a warning from environmental health and safety officers. Waste streams containing fluorinated organics should funnel into dedicated containers. Labs stay safer by arranging regular pick-ups and clear logs of what’s getting tossed.
Standard chlorine-based disinfectants don’t always work well against stubborn residues from aromatic boronic acids. Using solvent rinses, such as ethanol or acetone, often removes more material. A thorough wipe-down, even on surfaces that seem clean, reduces the risk of accidental contact weeks later.
Fact-Driven Solutions
Good habits keep people safe. Always follow the Safety Data Sheet (SDS)—it usually lists eye protection, nitrile or butyl gloves, and a sturdy lab coat as musts. For bigger batch work or weighing out larger quantities, a modern fume hood makes a real difference. After finishing, surfaces need careful cleaning and all waste should go in accordance with state and federal guidelines.
People also benefit from regular training updates. Industry standards evolve as more is learned about long-term exposure and environmental persistence. Encouraging quick reporting of accidents—without blame—helps everyone improve methods and catch hidden hazards sooner.
Driving Responsible Science
Staying alert and respecting every stage of a chemical’s use, from storage through disposal, not only protects individual health but also builds a more sustainable scientific culture. By taking real precautions seriously, from PPE to smarter waste management, chemists support safety and environmental protection with each experiment.