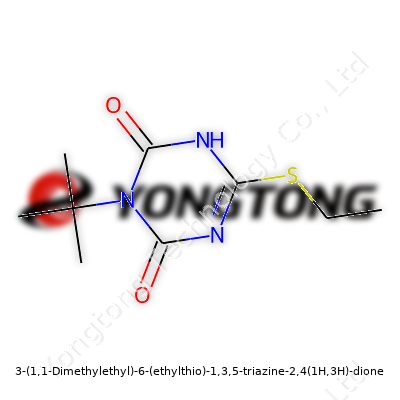
3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione: A Comprehensive View
Historical Development
Chemists started paying attention to the triazine family decades ago, and for good reason. Industries kept pressing for compounds with reliable stability and performance, and triazines stood out. With persistent needs in agriculture and materials science, 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione emerged from focused synthesis efforts during the late 20th century. Before the agrochemical boom, the original triazine backbone offered value in lab research, but modifications like tert-butyl and ethylthio groups brought a different set of functionalities. Teams in chemical plants saw this new analog as a way to enhance reactivity patterns and environmental resilience, especially since the classic dichloro-s-triazine compounds often underperformed in field tests. Now, its use often reflects years of synthesized wisdom—hard-earned knowhow from spanning decades of research, error, and practical feedback.
Product Overview
To someone who works with synthesis or crop protection, 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione means a solid option for advanced formulations. Labs produce it as a crystalline powder, often with a faint chemical odor, and package it in moisture-sealed drums. You recognize it by its off-white to pale yellow color, and once you have it in hand, the consistency stands up to challenging conditions. Formulators appreciate that its molecular tweaks give it a shelf life that surpasses many older analogs, and scaling up production no longer presents the mysteries it once did. Our sector values how the tert-butyl and ethylthio functionalities shift the compound’s performance in real-world settings—delivering improved selective action and outcomes compared to some relic chemistries.
Physical & Chemical Properties
This triazine analog brings more than just a chemical formula. Its melting point usually falls in the 110–115°C range, and it dissolves readily in many organic solvents, though it resists water. The bulk density and particle size demand regular verification because downstream processes depend on predictable flow and reactivity. Researchers notice its balance of molecular rigidity—thanks to the triazine ring—and flexibility from the bulky tert-butyl and ethylthio groups. Chemically, it resists hydrolytic degradation under neutral or slightly basic conditions, making storage and application straightforward if one avoids strong acids or oxidizers. These features push it ahead of less sophisticated triazines, offering a practical blend of stability and controlled reactivity.
Technical Specifications & Labeling
Technical data sheets highlight purity standards above 98% for this triazine. Supply chains demand batch records showing trace impurities, especially sulfur- and nitrogen-containing byproducts, as shifting regulations and product recalls shape the operational landscape. Labeling follows both the United Nations GHS (Globally Harmonized System) and country-specific protocols. Each package notes the standard product code, hazard class pictograms, recommended personal protective equipment, and disposal instructions. From my vantage point, transporters and handlers want crystal-clear documents, not just regulatory lingo—because on-site safety and downstream accountability depend on more than legal compliance; they require robust, commonsense detail on risks to human health and the environment. Labels must spell out incompatibilities with oxidizers or acids, so nobody learns through trial and error.
Preparation Method
Preparation of 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione usually starts with the classic triazine ring closure from cyanuric chloride, followed by regioselective nucleophilic substitution using tert-butylamine and ethylthiol. Controls on stoichiometry and temperature determine yield and selectivity, because incomplete conversion wastes reagents and creates waste that’s tough to manage. In pilot plants, engineers found that adding solvent at the right point curbs side reactions, and light exclusion matters to prevent degradation. Purification relies on crystallization rather than costly column chromatography, allowing scale-up without ballooning costs. Facilities committed to green chemistry look for solvent recovery and waste minimization, knowing that these measures cut costs and mitigate impacts from regulatory tightening.
Chemical Reactions & Modifications
The compound’s value often lies in its adaptability. I’ve seen chemists exploit the ethylthio group for targeted oxidation, converting it to sulfoxides or sulfones which tweak both solubility and biological performance. Under mild basic conditions, one can swap the tert-butyl amine for custom amines, opening the door to new analogs without reworking the entire synthetic route. These transformations support integrated pest management, specialty coatings, and even solid-state materials research. Direct halogenation or coupling with nucleophiles usually preserves triazine stability, but careful control of pH and reaction time is critical—a lesson learned from more than a few failed batches in industry and academia alike.
Synonyms & Product Names
In procurement sheets and academic papers, 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione goes by several names. “Tert-butyl ethylthio triazinedione” often shows up in Europe, while some suppliers list it as “N-tert-butyl-N′-ethylthio cyanuric diamide.” Legal registries globally maintain detailed synonym lists to avoid confusion: a mix-up in nomenclature or CAS numbers risks cross-contamination or the wrong warehouse shipment, with serious safety and financial consequences. The most trusted suppliers publish comprehensive synonym and trade name tables with each lot, and in the lab, I double-check every time to head off errors.
Safety & Operational Standards
Workers in synthesis and application settings respect this compound for both its utility and its hazards. Dust can cause skin and respiratory irritation, so fume hoods, N95 masks, and double-layer gloves are standard issue. Environmental Health & Safety teams keep up-to-date SDS available and build protocols for spills and disposal, reflecting experiences with similar triazines that taught the hard way about toxic releases. Regular training on chemical incompatibilities limits risks, and storage in cool, dry, well-ventilated spaces heads off unwanted reactions and hydrolysis. Audits check compliance because ER teams never want surprises in a real emergency, and site managers invest in exposure monitoring to keep risks as low as possible, not just within the letter of the law.
Application Area
The practical uses for 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione start with crop protection—custom pesticides designed to stay stable across seasons, with controlled breakdown that limits environmental persistence. The triazine core anchors selective herbicides, while modifications allow for fine-tuning uptake and plant metabolism rates. Materials scientists integrate it into specialty polymers and adhesives, benefiting from the compound’s backbone for mechanical strength and chemical resistance. Advanced coating formulas also profit from its unique blend of solubility and durability, showing up in everything from electronics to water-resistant packaging. In each field, users care about both performance and the costs of compliance, and teams track evolving regulations that may alter how and where the compound gets applied or restricted.
Research & Development
Researchers still chase new applications, often with public funding pushing for greener, less-toxic alternatives. Collegiate centers test derivative libraries for anti-microbial or anti-cancer activity, banking on the triazine scaffold’s historic versatility. Collaborations with private industry feed insights back to synthesis teams on what modifications bump up efficacy or cut negative side effects. Instrumental analysis labs keep working to pin down trace decomposition pathways and environmental breakdown products. These cooperative efforts deliver data peer reviewers and regulatory agencies both want, making translational R&D a daily routine. In my experience, multidisciplinary teams get more traction by pulling together field reports, lab results, and computational modeling so progress sticks—not just in journals, but in real-world settings.
Toxicity Research
For all its promise, research on toxicity matters just as much to everyone involved. Animal testing reports, environmental impact models, and worker exposure studies all shape risk assessments. The compound, like its relatives, can cause irritation on direct contact and brings aquatic toxicity concerns, pushing regulators toward tighter controls and new warning labels. Some rat models have shown chronic effects at high doses, pushing companies to invest in alternative in vitro toxicity tests and predictive modeling. Wastewater analysis now features as a key checkpoint in regulatory approval, and compliance professionals audit suppliers for REACH and TSCA filings. For personnel safety and environmental stewardship alike, data transparency and proactive sharing of new findings remain the most effective ways to cut risk and avoid future disasters.
Future Prospects
Three decades back, few people guessed this triazine derivative would anchor major advances in both agriculture and material science. Looking ahead, more sectors test it for use in advanced composites, organic electronics, and even as intermediates in pharmaceuticals. The main hurdle lies with regulatory adaptation and mounting pressure for green chemistry. Development teams focus on degradability and safer breakdown products, knowing that any compound’s long-term survival depends as much on its environmental profile as its scientific promise. Ongoing collaborations between manufacturers, researchers, and advocacy groups carry real weight, since policy and perception move markets just as much as technical data do. In this fast-moving landscape, only those outfits that invest in ever-safer and ever-more-transparent R&D will keep this and related triazines relevant for another generation.
Breaking Down the Purpose
3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione, known among growers and chemists as Terbutryn, gets poured into countless crop fields each year. Its job is simple but important: weed control. Farmers, co-op managers, and public park employees rely on it to help keep broadleaf and grassy weeds from crowding out their crops and green spaces. Terbutryn doesn’t belong to the list of household names like glyphosate or atrazine, yet it has stuck around for decades because it works well in cereals and other crops that need a reliable herbicide that won’t wipe them out along with the weeds.
Why Does It Matter?
Food production wraps around the tricky business of providing a steady, safe supply, and weeds throw a wrench into that work. Out in the field, weeds compete for water, sunlight, and nutrients. Losing the upper hand causes yields to fall, sometimes drastically. Terbutryn brings value by allowing crops like barley, oats, and wheat to get the jump on weeds. Reliable weed control has a ripple effect, letting growers invest less in hand weeding and replanting, while harvest quality and quantities go up. Those direct benefits filter down to buyers in the grocery store, keeping prices from swinging wildly.
Human Health and Environmental Considerations
Terbutryn isn’t without drawbacks. As with many synthetic herbicides, there are risks to water supplies and aquatic life. Studies find residues can build up in rivers—or even groundwater—if farmers use the product repeatedly over the years. The European Food Safety Authority and other agencies have flagged these risks, putting pressure on governments and industry groups to monitor and restrict Terbutryn’s use. Fish and amphibians prove particularly sensitive, showing negative effects at exposure levels far lower than those that harm people.
Communities living near agricultural land have raised concerns about potential links to chronic health conditions. These worries, valid or not, reflect more than just science; they also underscore how people feel about the chemicals entering their water or soil. Managing these conversations is every bit as important as reading lab test results, especially with the growing push for organic food and regenerative practices.
Improving Safety and Efficiency
Switching to precision agriculture offers promise. By using GPS-guided sprayers and digital mapping, applicators now place herbicides exactly where they’re needed. Less waste hits the ground, and the risk of run-off slides downward. Some seed companies also breed new crop varieties that out-compete weeds on their own or tolerate a wider range of herbicides, giving farmers more flexibility. Technologies like buffer zones, improved spraying methods, and better monitoring of environmental impact continue to shape how people use Terbutryn and other chemical weedkillers.
Few growers look forward to the day when resistant weeds make Terbutryn obsolete. Until then, the discussion should revolve around finding a safer balance: lowering the use of synthetic weedkillers, strengthening environmental safety nets, and making food production both dependable and affordable. Listening to growers and people living nearby goes a long way. Open dialogue, solid research, and investment in alternatives keep the system moving forward, without letting any one chemical take over the conversation.
Chemical Risk in Daily Life
Most people don’t think much about chemical names unless something goes wrong. 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione might not sound familiar, but that doesn’t mean it’s new or rare. Chemicals with complex names like this one often hide in agricultural products, industrial processes, or laboratory experiments. Stories about such substances rarely make the front page unless they trigger health and environmental alarms. For anyone working near this compound or eating food treated with it, the background information matters.
Looking at the Hazards
Experience goes to show that long chemical names can’t hide risks. 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione belongs to the triazine group, which has a track record in weed control and pest management. Atrazine sits in the same chemical family and has sparked global debates about safety. On principle, triazines target the nervous system of unwanted insects, but like many pesticides, these molecules don’t always stay where intended.
Reviewed data from the U.S. National Library of Medicine and the European Chemicals Agency shows that 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione can cause skin and eye irritation. Workers who handle the raw form without gloves or goggles run the risk of rashes or burning sensations. Inhaling the dust has links to headaches, breathing issues, and, at higher exposures, serious respiratory distress. Factories using this chemical typically require safety data sheets listing procedures for spills and emergencies.
Chronic and Environmental Concerns
Short-term contact, while unpleasant, isn’t the main source of anxiety. Toxicity concerns escalate with long-term or repeated exposure. Scientists pay close attention to the way triazine chemicals break down. Traces linger in soil and water, sometimes for months. Once there, these residues drift into groundwater and food chains. Regulators in Europe flagged related compounds for possible endocrine disruption, narrowing the scope for approved uses.
Cancer risk under lab conditions remains debated, but a handful of animal studies hint at connections to tumor growth and organ toxicity. The U.S. EPA often sets reference doses for these triazines, warning against chronic overexposure. Individuals working in agriculture or landscaping see the highest potential for risk if safety standards slip or protective equipment gets ignored.
Solutions That Make Sense
Paying attention pays off. Reading the safety sheets before handling any unfamiliar chemical produces better habits. Switching to closed application systems reduces direct exposure on farms and in factories. Personal protective equipment—goggles, gloves, and masks—keeps accidents rare, even if some see this gear as a hassle. On the government side, agencies update limits for airborne and waterborne residues as new science comes in. Environmental monitoring programs spot polluted sites early, and clear labeling on packaging helps consumers steer clear of unnecessary risks.
Finding alternatives with gentler environmental footprints still takes time and research. Manufacturers can reformulate products to lose persistent chemicals or swap to biologically-based pesticides. Education at every level—from field hands up to corporate decision makers—lowers misuse and accidental contamination.
Final Thoughts on Chemical Safety
3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione doesn’t sound like a household worry, but its risks are real and well-charted. Understanding facts beats relying on fear or guesswork. Responsible handling and stronger oversight stretch far in keeping both people and the environment safe as our chemical toolbox grows.
Everyday Challenges in Laboratories
If you’ve spent time in a lab, you learn fast: mistakes with chemical storage don’t wait for a convenient time to reveal themselves. A poorly sealed bottle can knock you flat with fumes. A forgotten compound in a sunlit corner can break down into something nobody wanted. I’ve seen researchers new to this overlook a humidity warning, only to find a chunk of clumped-together powder weeks later. These problems aren’t just annoyances—they threaten experiments, safety, and sometimes even careers.
Keeping Chemicals Where They Belong
Temperature swings cause real trouble. Acids stored above the recommended heat often corrode lids or create dangerous pressures. Organic solvents next to a heat source can evaporate much faster, making their concentrations unpredictable. Most labs keep temperature logs for a reason. A digital thermometer does wonders for peace of mind, and a well-marked, modern fridge dedicated for flammable materials prevents a whole spectrum of disasters.
Humidity leads to strange results. Hygroscopic powders suck water right from the air, turning precise dosages into mushy guesses. Working with pharmaceuticals, our team watched a batch move out of spec simply because someone stored it near the sink. The lesson landed hard: silica gel packs and dry cabinets earn their shelf space.
Don’t Underestimate the Light
Light isn’t always just light—UV exposure messes with a surprising number of chemicals. Colored bottles offer protection, and for especially sensitive compounds, a box lined with aluminum foil does more than you’d think. Once, a sample meant for an assay lost potency overnight on a bright bench. Since then, anything with a “light-sensitive” note gets moved straight to a designated dark drawer.
Preventing Cross-Contamination
Labels fade or peel, solvents share shelves with acids, and gloves touch more than they should. These small things matter. I once witnessed a mix-up where a technician grabbed an amber bottle from the wrong row—luckily, another tech caught it in time. Clear, fresh labels and segregated storage stop most of these problems. Color-coded tape worked best in our busy space, backed up with checklists that anyone could follow even in a hurry.
Solutions and Smarter Practices
Changing old habits isn’t always simple. The research community keeps pushing for better training, but experience counts. Running refresher sessions every few months beats relying on memory alone. Simple tools—like checklists, secondary containment trays, and quick-access safety data sheets—turn good intentions into safe routines. It’s good practice to double-check expiry dates, and once a bottle looks off—cloudy, crusted, bulging—nobody waits to ask questions. Disposal keeps the lab safe and the data clean.
Proper storage and handling go further than guidelines on paper. They protect health, save costly reagents, and keep experiments honest. From color-coded bins to dedicated coolers, these chores pay off every day. No fancy system replaces watchful eyes and pride in a well-run workspace.
Looking at Environmental Impact
People have real concerns around how products shape the environment around us. In my own years living near farmland and industrial zones, I've seen how runoff or emissions remind communities that product choice matters. Sometimes, substances leak into groundwater or hang in the air longer than expected. Chemicals can throw off the natural balance, harming everything from soil microbes to freshwater bugs.
Take the past few decades. Reports have linked some manufacturing ingredients to air quality dips or fish kills downstream from processing plants. Runoff doesn’t always stay put. Small rivers carry it miles away, affecting both crops and wildlife people rely on. Even low doses accumulate over time and can spark headaches for farmers and health officials alike. The World Health Organization has tracked growing concern about certain persistent chemicals entering global water supplies, calling for tighter monitoring.
Regulations Keep Evolving
Most countries require products to clear strict safety and labeling rules. In the U.S., the Environmental Protection Agency (EPA) enforces tough restrictions on anything suspected to linger or spread into surrounding areas. Companies need to measure potential risks, present data, and follow field tests before selling at scale. If a new ingredient shows up in soil or water, departments like the USDA or FDA can step in and halt distribution. On the global stage, Europe’s REACH Regulation forces businesses to identify hazards early and share findings both up and down the supply chain.
Real life doesn’t always match regulations on paper. Enforcement gaps pop up when people cut corners or when agencies run short on funding and staff. Even so, things look better than before: modern tracking tech, satellite imagery, and tighter reporting laws catch incidents far earlier than decades ago.
Finding Better Solutions
Plenty of people oppose “one-size-fits-all” mandates, but my experience tells me that careful reforms make sense. Take buffer zones: lining waterways with natural barriers traps runoff, preventing pollution at the source. Incentives for greener alternatives, plus grants for research, can turn high-impact products into safer ones. More universities are teaming up with local governments to swap in less toxic options without breaking the bank.
Consumers help push progress, too. Demanding transparency nudges companies to test their products better and disclose ingredients on packaging. Some cities now require QR codes or online databases showing exactly what goes into products, and how each has been checked for impact.
Long-Term Choices Shape the Future
Decisions about how products touch the land, water, and air always reach beyond business. Everyone—makers, buyers, regulators—shares a stake in outcomes. My own neighbors started meeting more often after local fish warnings, and their grassroots input helped shape fairer, tougher safety checks. Experience tells me that change often starts small, through shared responsibility and consistent follow-through.
It’s not about fighting over new rules or defending status quo. It’s about keeping people and places safe, building trust, and aiming for smarter alternatives that let work and nature fit side by side.
Pushing Beyond the Bins: The Realities of Chemical Waste
Chemical names rarely tell the full story. With 3-(1,1-Dimethylethyl)-6-(ethylthio)-1,3,5-triazine-2,4(1H,3H)-dione, most folks outside of a lab wouldn’t recognize the name, but its presence in industrial and research settings demands careful handling, especially when it’s time to part ways with it. Tossing it into the trash is never on the table. Ignoring the risks that come with improper disposal puts water, air, and soil in the line of fire, not just in theory, but with skin rashes, toxic air, and fouled waterways in real towns and neighborhoods as the fallout.
Incineration: The Gold Standard with Its Own Set of Hurdles
High-temperature incineration stands out as the top choice for a reason. At certified hazardous waste facilities, trained crews and robust systems break down the compound at temperatures that knock out complex molecules. The result? Safer end products like carbon dioxide, water, and simple salts. Not every region can boast access to a proper incinerator, though, and hauling wastes to such centers racks up its own costs and risks. Cutting corners here means risking incomplete breakdown, and that’s a recipe for toxic emissions or stubborn residues. I once visited a community near a low-standard burn site, and folks there got a front-row seat to what happens when cost-cutting trumps safety: chronic coughs, strange smells, and a string of unexplained illnesses.
Chemical Treatment: Getting the Details Right
Some labs and plants use chemical neutralization. This method means using other chemicals, often basic or oxidizing agents, to break the triazine ring or tip the sulfur off its perch. But it’s not like stirring sugar into coffee. Proper doses, complete mixing, and thorough monitoring count for everything. Miss a step and you might create more hazardous by-products or leave partly-reacted junk behind. Every waste manager I’ve ever learned from stressed the track record of near-misses caused by sloppy setups—leaks, off-gassing, and worst of all, unpredictable leftovers. In my city, a poorly managed batch led to an emergency evacuation of a university building. That nightmare made it clear: chemical treatment saves money and time only if it’s run by trained hands following tight protocols.
Secure Landfill: The Last Stop—And the Most Controversial
For waste that resists easy breakdown, secure landfill disposal becomes the default. Special lined pits and covers prevent chemicals from leaching into groundwater. Some environmental watchdogs keep close watch, since failures here carry serious consequences. No system is bulletproof, and once the cap goes on, whatever lies underneath sticks around for decades. I live near an old landfill that once accepted chemical waste, and groundwater monitoring still takes place monthly, with warnings posted for people relying on wells. There’s always worry in the air about what future storms or earthquakes could let loose.
Better Paths Forward
Safer disposal methods must keep pace with new chemicals and growing volume. Investing in more local incineration sites, coupled with tighter regulations and real oversight, closes the gap for communities far from main hubs. Providing practical training for waste managers—shoulder to shoulder, not just online—raises the bar for safe work. Some regions are piloting “green chemistry” that limits hazardous by-products from the start, a smart move for industries looking down the line at mounting disposal bills and stricter laws. People living near waste routes and landfills can’t all move away, so smart policy and investment now pay off in fewer headaches later. Facing the truth about chemical waste means demanding the best systems, skilled workers, and the right priorities, not just for labs, but for every household that shares the water and air.