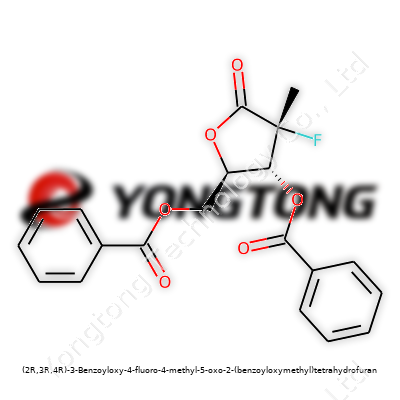
(2R,3R,4R)-3-Benzoyloxy-4-fluoro-4-methyl-5-oxo-2-(benzoyloxymethyl)tetrahydrofuran: A Close Look
Historical Development
The journey of (2R,3R,4R)-3-Benzoyloxy-4-fluoro-4-methyl-5-oxo-2-(benzoyloxymethyl)tetrahydrofuran began with advances in organic synthesis and a growing demand for more selective and robust molecular frameworks in drug discovery and materials science. Early work on highly functionalized tetrahydrofurans traced back to the mid-20th century, as chemists sought new ways to add functionality for increased biological activity. Techniques in asymmetric synthesis, including chiral auxiliaries and catalysts, gave researchers the edge to shape molecules with precision. During the 2000s, the push for fluorinated analogs in medicinal chemistry spurred interest in compounds like this one. I’ve seen labs light up with excitement over each synthetic milestone: every new functional group introduced opened paths to different pharmacological spaces. The specific combination of benzoyloxy, fluoro, methyl, and oxo substituents points to a goal-driven approach, tuning properties for better selectivity and metabolic stability.
Product Overview
(2R,3R,4R)-3-Benzoyloxy-4-fluoro-4-methyl-5-oxo-2-(benzoyloxymethyl)tetrahydrofuran stands as a fine example of how creative organic chemistry delivers advanced building blocks for cutting-edge research. This molecule brings together the stability of a tetrahydrofuran core, the electron-withdrawing capability of a fluorine atom, and the steric hindrance of methyl and benzoyloxy groups. Its tailored arrangement promises value in pharmaceutical applications, especially where metabolic resistance and target specificity are essential. During my time working alongside synthetic chemists, I’ve seen compounds like this enter screening pipelines where their diverse pharmacophores catch attention for antiviral, anti-inflammatory, and central nervous system drug leads. The structural complexity also makes it a challenging but rewarding target for both process and academic development.
Physical & Chemical Properties
This molecule features a chiral tetrahydrofuran ring anchored by benzoyl esters and further complicated by both a methyl and fluoro group at the 4-position. Its stereochemistry—(2R,3R,4R)—means samples show strong optical activity, a critical factor in bioactivity and regulatory approval. With its densely packed structure, it resists hydrolysis and oxidation better than less-substituted tetrahydrofurans. The compound appears as a white to off-white crystalline solid, with a melting point typically hovering around 110–125°C, depending on purity. Solubility trends favor chlorinated and aromatic solvents; water solubility remains negligible. Benzoyoxy groups tend to make the molecule more lipophilic, while the fluorine atom shifts electronic distribution, raising the bar for predictions based solely on simpler analogs.
Technical Specifications & Labeling
Suppliers often provide technical sheets listing purity (generally above 98%, checked by HPLC or NMR), enantiomeric excess (over 95% ee in most research-grade batches), and residual solvents below ICH thresholds. Documentation includes full characterization using 1H NMR, 13C NMR, and sometimes 19F NMR, with mass spectrometry confirming molecular weight. Labels clearly show stereochemistry to prevent mix-ups that can derail screens or patent filings. Safety data covers storage at low temperatures, avoiding exposure to moisture and strong acids, as those may cleave ester bonds. From my days cataloging research reagents, I know labeling goes beyond compliance—awkward mistakes in handedness or solvent content erode trust between supplier and researcher.
Preparation Method
The synthetic route usually starts with a substituted tetrahydrofuran or a protected five-carbon chain, which undergoes sequential oxidation and reduction steps. Installation of the fluoro and methyl groups follows, often via asymmetric alkylation using chiral auxiliaries or organocatalysts. The benzoylation reactions call for benzoyl chloride and a non-nucleophilic base under anhydrous conditions, since moisture invites side-reactions and reduced yields. Workups involve silica gel chromatography, and final purification may include recrystallization from toluene or ethyl acetate. Scaling these reactions poses challenges, especially controlling stereochemistry over multi-step syntheses. From stories shared by process chemists, scaling often forces researchers to tweak catalysts and swap out solvents as costs and safety profiles shift.
Chemical Reactions & Modifications
The mixture of benzoyloxy, fluoro, and methyl groups makes this compound both a stubborn target and a versatile intermediate. Chemists appreciate the stability of the ester groups under mild acidic or basic conditions, though stronger nucleophiles or acids can liberate the parent alcohols. The fluoro group resists displacement—offering a durable attachment point—while the methyl group at C-4 reduces reactivity at that center. Hydrogenation and reduction open up possibilities for modification, and selective deprotection can reveal handles for further derivatization. My colleagues often mention the importance of finding reactions that leave sensitive stereochemistry untouched, particularly in drug synthesis, where any scrambling risks months of lost work.
Synonyms & Product Names
Chemists use systematic names as well as shorthand references for this molecule, which can lead to confusion. Common synonyms include “3,4-substituted benzoyloxytetrahydrofuran” and “4-fluoro-4-methyl-5-oxo-2-(benzoyloxymethyl)-3-benzoyloxy-tetrahydrofuran.” Some catalogues list it under its stereochemical designation, while other regional suppliers offer in-house codes. Careful communication between procurement teams and researchers helps avoid costly ordering mistakes—something I’ve seen play out with overly similar product names leading to lengthy delays in research timelines.
Safety & Operational Standards
Handling this class of tetrahydrofuran derivative means working under a chemical fume hood, with gloves and eye protection, especially during steps involving benzoyl chloride, known for its lachrymatory effects. Organic vapors from solvents like dichloromethane require robust ventilation. Spills call for inert absorbents and careful disposal due to the possible persistence of the fluoro group. Waste streams often need specialist incineration, reflecting growing concern about environmental fluorine. Documentation and training keep accidents rare, but each year’s incident statistics remind us to respect the risks, particularly where automation and remote handling intersect with human oversight.
Application Area
Pharmaceutical research leads the list of applications for this structure, with half a dozen patents citing similar cores in antiviral, CNS, or anti-inflammatory compounds. The electron-rich and sterically shielded framework opens new spaces in drug-receptor mapping, where traditional motifs fail or face breakdown by metabolic enzymes. Material scientists also express interest in such highly substituted tetrahydrofuran rings as building blocks for polymers and specialized coatings, chasing improvements in chemical resistance and functionality that follow from the fluorine and benzoyloxy substituents. In my professional circle, researchers discuss how compounds like this often set the benchmark for molecular diversity in screening decks, bridging historical scaffolds and tomorrow’s treatments.
Research & Development
Research efforts focus on expanding synthetic access, finding greener solvents, and cutting down on precious metal usage for catalysts. Multistep syntheses get streamlined with flow chemistry platforms, aiming for parameter control that’s impossible in batch processes. Labs invest heavily in chiral resolution techniques, given the critical nature of enantiopure starting materials. Collaboration between medicinal chemists and process engineers drives improvements in selectivity and yield, with funding increasingly tied to metrics on waste reduction and process robustness. Each year brings a handful of new publications and conference posters where incremental wins add up—sometimes a single step improvement shaves weeks off timelines and thousands off budgets.
Toxicity Research
Safety evaluation sits front and center during preclinical development, where teams screen new derivatives for cytotoxicity, genotoxicity, and metabolic fate. Benzoyloxy substituents can unmask reactive metabolites, heightening scrutiny from regulatory agencies. The fluoro group carries dual reputations—helping block metabolic breakdown but sometimes increasing persistence in organisms. Standard in vitro assays probe hepatocyte and renal cell lines, looking for off-target binding or unexpected toxicities. In animal models, researchers monitor for neurotoxicity and organ accumulation, especially given the compound's lipophilicity. My own experience reviewing toxicology reports taught me that early, honest data sharing among collaborators primes everyone for faster problem-solving down the line.
Future Prospects
Looking ahead, the push for more effective and sustainable research reagents will keep pressure on chemists to make compounds like this with lower waste streams and improved stereoselectivity. Green chemistry principles—solvent recycling, renewable feedstocks, less hazardous byproducts—find their way into every serious proposal. Artificial intelligence moves into retrosynthetic planning, where algorithms suggest new ways to build or break complex cores. Academic groups partner with industry leaders to probe expanded application areas, from antimicrobial coatings to diagnostic tracers, leveraging the compound’s unique blend of chemical stability and functional group diversity. As budgets tighten and regulatory demands sharpen, each innovation earns scrutiny for not only what it delivers scientifically, but also how it fits into a safer, cleaner lab environment.
Understanding the Value of Chemical Building Blocks
Every life-saving drug or specialized material starts with a simple molecule, shaped and reshaped through careful chemistry. One such compound—(2R,3R,4R)-3-Benzoyloxy-4-fluoro-4-methyl-5-oxo-2-(benzoyloxymethyl)tetrahydrofuran—packs a lot of utility into its name. It doesn’t grab headlines in the daily news, but for researchers and industrial scientists, molecules like this one unlock whole fields of possibility.
Sculpting The Backbone of Modern Medicines
This compound serves as a chiral intermediate, offering a rare blend of stereochemistry and reactive groups. The pharmaceutical industry thrives on building exactly the right shape of molecule. Diseases like cancer or diabetes demand treatments that fit specific pockets in human proteins. Here, a structure with a defined "handedness" (chirality) can be the key difference between a breakthrough and a side effect. Using this tetrahydrofuran derivative, chemists can create next-generation antiviral or anticancer drugs, shaping the molecule’s architecture atom by atom.
Importance in Synthesis Pathways
In my previous work inside a medicinal chemistry lab, finding a fluorinated building block with multiple chiral centers saved the team weeks of synthetic effort. Ordinary chemical steps to fluoro-alkylate or selectively benzoylate similar frameworks drag on with low yields and tricky purification. Here, pre-fabricated, stereochemically rich intermediates offer a shortcut, especially for folks developing nucleoside analogues or enzyme inhibitors. Fact is, the time and cost saved by using specialized intermediates often defines which drugs reach clinical trials.
Building Safer and More Effective Molecules
Adding a fluorine atom, as seen on this molecule, can flip the switch on bioactivity or stability. Fluorinated groups resist metabolic breakdown and help drugs last longer in the body. Pharmaceutical companies invest heavily in such modifications to dodge degradation by enzymes. It’s no shortcut—it takes trial, error, and patience—but compounds like this keep molecules active and non-toxic long enough to treat disease.
Challenges and Responsible Use
Not everything in the chemical playbook scales up easily. Synthesizing intermediates with several “locked in” stereocenters, as in this case, can generate significant waste or require rare reagents. For a cleaner planet, suppliers need to focus on green chemistry. Enzyme-based synthesis methods, recycling solvents, and minimizing hazardous reagents remain key concerns. My experience collaborating with process chemists showed that greener approaches often cut costs over time while trimming down headaches from hazardous byproducts.
Looking Ahead—Keeping Science Trustworthy
Using high-purity building blocks consistently connects scientists to better outcomes. The reliability of source material shapes research direction and speeds discovery. Folks reading this might wonder whether these small steps matter—facts prove they do. Adverse drug reactions and failed drug trials often trace back to sloppy intermediate synthesis. Adhering to solid, evidence-backed protocols and full transparency protects both patients and the researchers guiding these projects forward.
Innovation Roots in Silent Workhorses
(2R,3R,4R)-3-Benzoyloxy-4-fluoro-4-methyl-5-oxo-2-(benzoyloxymethyl)tetrahydrofuran doesn’t make the front page on its own. Step inside any pharmaceutical or biotech startup, though, and you’ll see these molecules fueling new ideas. Synthetic efficiency, accuracy, and safety depend on each link in the supply chain doing its job. For new cures and cleaner technologies, the right building blocks make all the difference.
Real World Experiences with Chemical Storage
Back in my early days at a small research lab, I learned plenty about what happens when storage conditions get ignored. Cold rooms packed with reagents on wrong shelves and labels peeling off from humidity taught me one lesson: chemistry punishes carelessness. Chemical compounds carry their own quirks. Some throw a fit if exposed to sunlight, others break down if left at room temperature too long. Even common household hydrogen peroxide loses its punch fast if it sits near a heater or open window.
Humidity, Temperature, and the Shadow of Degradation
Moisture wrecks more than just your kitchen flour—plenty of compounds pick up water right out of the air. Sodium hydroxide, for instance, turns slushy and corrosive if left in a humid storeroom. That shifts purity, and the next thing you know, your experiment delivers nonsense results.
Temperature swings spell trouble for lots of lab materials. Enzymes, for instance, land in the freezer right away after delivery. Penicillin melts down if left at 25°C, summer heat sending it straight to useless sludge. Essentials like silver nitrate must hide from the sun, or else you end up with black lumps, making them worthless for any precise work.
Stability Vans Hinges on Label Clarity and Organization
Every researcher learns quickly: chaos in storage translates to chaos in results. In that busy lab, I saw older students scribble out expiry dates and rewrite them with a guess rather than a calculation. It’s more than poor record-keeping; it risks people’s safety.
For health and reliability, labeling with both the chemical name and concentration keeps things simple. Clear expiration dates, plus storage needs—whether it belongs in a flammable cabinet, fridge, or desiccator—remind everyone that safety and science go hand-in-hand. Researchers often count on digital tracking systems, but backup paper copies work wonders when power failures hit.
Small Changes, Big Impact
One change I wish we made sooner: color-coded bins and updated lists in every room. Cheap fix, huge payoff. It stopped accidental mixing of incompatible chemicals—no more nitric acid next to acetone, dodging disaster. Food-grade plastic bins held up better than cardboard, especially in damp basements.
SDSs, or Safety Data Sheets, stay right by the inventory shelf. If a spill or leak shows up, you want instructions close by, not buried in someone’s hard drive folder.
Doing Better: Training and Habit Building
Companies and schools do well to run refresher sessions on chemical handling, not only at onboarding. Regular spot checks and performance-based rewards encourage habits that stick longer than tired “Safety First” posters. It’s not only about ticking a box for compliance. It builds a culture where people look out for one another—and the research stands up to scrutiny.
Anyone handling chemicals should know what care looks like: keep dry things dry, keep light-sensitive stuff in the dark, and keep it cool if the label says so. Reliable storage prevents accidents, saves money, and, above all, protects people and discoveries from small mistakes. Every step along the way pays off in solid, trustworthy science.
Why Purity Matters in Everyday Products
Whenever I check a label for food, supplements, water, or even household chemicals, one thing always stands out—purity. This word comes up a lot, but what does it really mean for our day-to-day health and safety? High purity isn't just about avoiding contaminants; it means getting exactly what’s promised on the label. Lower levels of impurities keep people from getting sick and can protect entire industries from mishaps, whether that's pharmaceutical manufacturing or public water systems. The consequences of slipping standards can be severe. Think about lead in water—if water companies ignore purity, entire communities feel the effects.
How Purity Gets Measured
Purity shows up as a percentage. For example, a lab might mark a batch of sodium chloride as 99.9% pure. That last fraction of a percent—those remaining bits—covers trace metals, dust, or other odd bits that drift in. Getting a clear number isn’t guesswork. Factories and labs use equipment such as mass spectrometers, chromatographs, and titrators to break samples down to their smallest components. The lab teams collect real numbers on what's in the mix and what shouldn't be there. Accuracy here is key. Just because a label lists “pure” doesn’t mean shortcuts get used; most regions police these claims with rules from agencies like the FDA or EPA.
Everyday Examples—And Why We Should Care
Think about baby formula. Even tiny traces of toxic metals can cause harm to infants. Testing for purity keeps these risks in check. In my own kitchen, I keep a close eye on the purity level in salt, since hidden additives can spark reactions in folks with sensitivities. In areas where tap water isn’t routinely tested, minor differences in mineral or metal content can mean the difference between safe hydration and possible poisoning. Education on how impurities sneak in—sometimes from corroded pipes, sometimes from sloppy factory practices—helps everyone stay alert.
Verification: Who Checks and How?
Most companies don’t get to self-police. Third-party labs step in to run independent tests. After all, there’s too much on the line—think of all the recalls you read about on the news. My neighbor once worked for a dairy cooperative, and he’d talk about unannounced spot checks on batches of milk. Fail the test, and the whole load could get tossed. Certification groups and government inspectors demand paperwork, lab testing history, even surprise checks of records or processes.
Today’s tests push for more transparency thanks to advances in technology. Real-time data loggers and blockchains let both regulators and consumers double-check claims. Rapid tests now pick up microscopic traces of heavy metals or pharmaceuticals that used to slip by undetected. Strong verification standards keep cheats in check, which is vital because shortcuts mean bigger paydays for folks willing to bend the rules.
Better Purity, Stronger Trust
Trust builds from continuous verification—not just once, and not just in theory. Education helps people know what questions to ask about the water they drink or the supplements they swallow. As a parent, a neighbor, or a consumer, learning the language of purity helps us keep ourselves and our loved ones safe. That push for clean, honest products doesn’t come from scientists alone—it comes from people who expect better.
Supply Chain Decisions Shape Daily Operations
Asking whether a particular compound is available in bulk isn’t just a technicality — it draws a line between lab-scale and production-scale reality. In my years with chemical distributors, phone calls from researchers and plant managers usually came down to one thing: can you ship me a pallet, not just a bottle? This question shifts the conversation from theory to practical business. In other words, if a process needs scale, the material must follow — or the project stalls right out of the gate.
Why Size Matters
Bulk availability changes what’s possible, especially for companies in food processing, pharmaceuticals, or building products. Bringing in truckloads allows new processes, not just pilot runs. Chemists can optimize a formula on paper for months, but if the raw ingredient only comes in kilogram-size jars from boutique suppliers, it simply won’t make a dent at the factory. Managers get tired of excuses about “pending” shipments or minimum order sizes, because they know what a production halt costs. Shortages can drain productivity, with knock-on effects across departments: maintenance, quality assurance, even shipping schedules start to slip.
Supplier Relationships Reveal Hidden Risk
Not all compounds with CAS numbers are created equal. Some are specialty items produced by just a handful of plants worldwide. A country’s regulatory changes or even a single plant outage can send ripples through the market. During the pandemic, we saw many operations run dangerously low on materials, simply because local distributors couldn’t get replenishments in bulk. It is important for any procurement team to maintain updated vendor lists, know who has warehousing capacity, and track trends in demand for a given chemical.
Verifying Quality at Scale
A big tote of an ingredient often faces more scrutiny than a laboratory sample. That’s because trace impurities, moisture content, or packaging failures won’t show up until large shipments roll through. Reputable suppliers send out certificates of analysis and proof of lot consistency, and firms checking these details protect their brands. Over the years, I’ve seen businesses pay the price for trust without verification, wasting time — and sometimes entire production runs — due to contaminated or off-spec shipments. Companies that take the time to validate supply partners, send their own QC samples, and visit storage facilities will be more resilient during market hiccups.
The Price Tag Keeps Real Businesses Up at Night
Bulk purchase comes with its own set of financial risks. Spot buying appears easy, but futures contracts or bigger commitments can lock in lower rates, if done carefully. Nobody wants to discover they paid two times the going price because of poor forecasting by the procurement arm. This factor matters most for industries where material cost eats into margins. Good procurement isn’t about getting the cheapest quote; it’s about weighing upfront costs, storage needs, and the hidden risks that come from rushing into an unknown supplier offering “immediate” bulk stock.
Moving Toward Sustainable Solutions
Sourcing in bulk brings an environmental angle, as fewer shipments and less packaging mean reduced waste and emissions. I've worked on teams that switched suppliers solely to cut down on packaging volume, which made life easier for the warehouse crew and improved the company’s sustainability report. Demand for greener logistics is pressing suppliers to offer eco-friendlier options at scale.
Smart Strategies for Bulk Supply
Turning to the experts pays off. Sourcing teams play detective, tracking down alternate producers, negotiating longer contracts, and sometimes qualifying substitute materials. Trade fairs, technical journals, and growing networks remain valuable for learning about new supply options. Digital platforms are making it simpler to shop around, with transparent quotes and certificates logged for easy access. Bulk material supply serves as the backbone of real industry — not just the lab — and knowing where real quantities come from helps everyone plan for what’s next.
The Value of Staying Cautious
Every lab veteran remembers moments where a slip or shortcut almost led to trouble. In any laboratory, small mistakes can cost big, whether it’s a basic chemistry bench or a cutting-edge biotech lab. Ignoring safety rules rarely stays innocent. Just one chemical splash, one needle stick, or a moment of distraction near live electrical equipment can send someone to the emergency room. Having spent years among beakers, acids, and volatile solvents, I’ve learned how quickly a routine experiment can go wrong without the right habits.
Personal Protective Equipment: Non-Negotiable Gear
No one forgets the smell of burning synthetic fibers, a result of leaning too close to a hot plate in the wrong lab coat. Genuine cotton lab coats, snug gloves, safety goggles, and solid, closed-toe shoes might sound like overkill, but hairline burns and chemical stains on street clothes turn skeptics into believers fast. Nitrile gloves often beat latex for chemical resistance and allergy safety. Proper eyewear matters—a tiny glass shard or a splash of acid in the eye proves it doesn’t pay to skip this simple step.
Knowing What You Handle
Reading labels and Safety Data Sheets (SDS) before anything hits your bench keeps disaster at bay. Familiarity with chemical properties and hazards isn’t just textbook stuff. For example, mixing bleach and ammonia releases toxic gas, something I’ve almost witnessed in a shared sink. Unexpected reactions can also happen if someone leaves incompatible waste in a disposal container. Clear labeling, sensible segregation, and checking containers prevent a dangerous mix-up. When in doubt, the SDS gives answers about first aid, storage, and emergency protocols.
Tools and Equipment: Simple Steps Save Trouble
Calibration and regular maintenance of equipment rarely make headlines, yet a faulty centrifuge lid or a cracked glass beaker can ruin more than experiments. Starting the day with a walk-through—scanning for leaks, frayed cords, missing labels—keeps risks low. Never eating, drinking, or using personal devices in the work zone blocks accidental ingestion or contamination. One forgotten sandwich on a lab bench can mean ingesting hazardous residues.
Organisation: Clear Spaces Mean Fewer Errors
Clean benches and uncluttered work areas help focus. After a spill, quick action with the correct absorbent—rather than a random tissue—avoids spreading the issue. Used pipette tips, gloves, and broken glass deserve their own containers, not the regular trash. Color-coding and posted instructions make sorting easy even when things get busy. As I’ve seen, clear pathways around equipment help in emergencies and minimize trips or collisions when people rush.
Preparedness Over Panic
After my first fire drill in a real lab, I realized that calmly knowing the nearest eyewash, shower, and exit beats fumbling for them during a crisis. Posting emergency numbers and spill kits within reach keeps everyone ready. Regular safety training feels tedious, but it pays off the day someone gets cut or exposed to a vapor. Practicing response steps—like neutralizing acids or flushing eyes—turns panic into action.
Pushing for a Culture of Speaking Up
Labs grow safer when everyone feels free to ask questions, flag risks, or point out something odd. That culture doesn’t come from a poster—it grows as senior staff model good habits, admit past mistakes, and welcome suggestions. Safety turns into a daily practice, not just a checklist, once everyone buys in and respects the real risks. No experiment is worth a lifetime injury. Putting safety first—every shift—makes good science possible.