2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid: A Deep Dive Into Utility and Research
Historical Development
Chemists found themselves needing solutions that could reliably maintain stable pH in a range of biological processes, and that's how 2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid (more commonly known as TES) entered laboratory vocabulary in the late 20th century. Research papers from the 1960s onward highlighted complications in enzyme work traced back to poor buffer performance. Labs craved reliability, simplicity, and safety. Scientists rolled up their sleeves and crafted organic buffers, with TES becoming one of the essential pieces of that toolkit. The story of its rise tracks closely with a general shift in life science toward better control of experimental conditions, less interference, and more predictable results. You won’t see the same degree of specificity or depth in older literature, but that also explains why so many published protocols embedded TES so quickly, finding in it a balance between compatibility and chemical resilience.
Product Overview
TES landed on the bench as an effective zwitterionic buffer ideal for physiological pH, bridging a gap between older buffering systems and the precise demands of cell biology, protein chemistry, and even diagnostic work. Its widespread adoption speaks volumes not through advertising, but through thousands of citations in peer-reviewed literature across molecular medicine, protein crystallization, and diagnostics. This kind of organic compound didn't emerge as a commercial juggernaut through branding, but through a relentless focus on practical outcomes – tolerating ionic strength changes, not poisoning enzymes, and offering comfort to researchers skeptical of every unfamiliar chemical they handled.
Physical & Chemical Properties
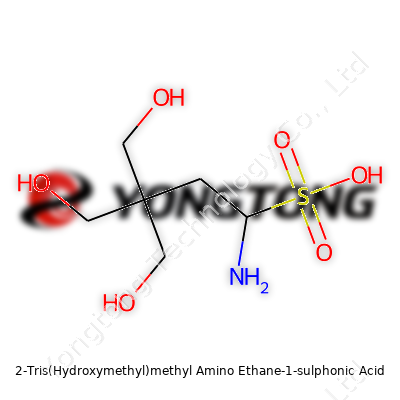
TES appears as a white crystalline powder, easily soluble in water, creating clear solutions that resist foaming or clouding. Its molecular weight sits at 229.27 g/mol, which helps in preparing precise buffer solutions without a mess of byproducts. Its pKa value hovers near 7.4 at 25°C, matching closely with human physiological conditions and making it friendly for blood-based or tissue-based studies. With a chemical formula of C6H15NO6S, TES stays stable under regular lab conditions, showing resistance to breakdown from light or minor fluctuations in temperature. Its presence stabilizes investigations involving metal ions because it doesn’t chelate most important cations, which means researchers can explore enzyme kinetics or protein structures with less worry about hidden interference.
Technical Specifications & Labeling
Bottles of TES typically arrive labeled with batch-specific purity (expect ≥99%), moisture content, and precise pKa data obtained under calibrations performed at 25°C. Labels note whether the buffer passes heavy metal testing, bacterial endotoxin burden, and UV absorbance profiles at 260 nm and 280 nm, which is vital for protein and nucleic acid work where even a slight impurity can send experiments astray. Storage guidance generally recommends tightly closed containers at room temperature, away from acids or oxidizers. Analytical-grade TES targets labs needing reliable, impurity-free results, and paperwork tracks certifications of analysis. With REACH registration for European Union safety compliance and compatible labeling for global markets, the compound sails across customs to thousands of research settings.
Preparation Method
Synthesis begins with tris(hydroxymethyl)aminomethane and reacts it with ethane sulfonic acid chloride using a base such as sodium hydroxide, in aqueous or mixed aqueous-organic media. After neutralization and purification by recrystallization from water or alcohol-water mixtures, the result is the clean, usable TES powder. This process yields predictable batches with standard purity levels, while sharp eyes stay peeled for byproducts or contamination with other sulfonic acids. Quality control teams analyze each lot for appearance, solubility, and freedom from foreign particulates. Small-scale synthesis matches textbook procedures, while manufacturers scale routes up for industrial supply lines serving universities, pharmaceutical developers, and diagnostic kit makers.
Chemical Reactions & Modifications
TES holds up well to autoclaving and doesn’t participate in spontaneous side reactions during ordinary use. Chemically, its primary amine is the most reactive handle, allowing conjugation to enzymes, proteins, or fluorescent labels through carbodiimide or NHS-ester chemistry, offering security for immobilization and tracking strategies. At elevated temperatures or extreme pH, decomposition can entail cleavage of the tris(aminomethyl) group or desulfonation. Its stability against oxidation stands out, letting it hold its ground in reactions involving ascorbate or iron. The structure prevents chelation with magnesium or calcium ions, which often preserves enzyme activity, unlike certain phosphate buffers notorious for sapping life out of a reaction.
Synonyms & Product Names
TES appears across catalogs as 2-Tris(hydroxymethyl)methylaminoethanesulfonic acid, N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid, or simply as Ethanesulfonic Acid, 2-[(2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]-. Commercial brands sometimes prefix the compound CAS number (7365-44-8) for global traceability, but researchers and technicians mostly ask for “TES buffer” or “TES sodium salt” for quick orders. Mixing up labels can cause confusion, but CAS-indexing usually prevents accidental swaps with similar buffers like MES or HEPES.
Safety & Operational Standards
With its low acute toxicity, TES ranks among the safer chemicals seen in biological research labs. Material safety data sheets indicate mild eye or skin irritation potential, requiring routine gloves and splash goggles during mixing and aliquoting. Accidental ingestion or inhalation should be treated as with any lab chemical – wash exposed areas, seek fresh air, and consult a physician in case of discomfort. Disposal goes with general buffer chemicals, and TES lacks the combustion or volatility risks that haunt other organic buffers or volatile amines. Storage guidance highlights the need for dry, well-ventilated spaces to avoid caking and clumping, but not more – this buffer doesn’t demand hazmat suits or special ventilation. Its environmental release profile remains favorable, showing low bioaccumulation and rapid breakdown under sunlight or wastewater treatment.
Application Area
Every life scientist working on proteins, cell cultures, or blood products encounters problems with unstable pH. TES steps in as a reliable buffer in cell biology, supporting in vitro tissues without triggering odd metabolic shifts. As a medium component, it holds pH lanes steady during electrophysiological studies. In clinical chemistry, it maintains blood sample integrity and supports accurate readings of calcium, magnesium, and enzyme activities. Diagnostic kit developers trust TES to stabilize enzymatic reactions during shelf storage. Even outside biomedicine, TES gets used where metal-sensitive processes unfold, such as bioremediation or water testing, thanks to its resistance against forming unwanted complexes.
Research & Development
Research teams exploring protein folding, ligand binding, and enzyme catalysis depend on buffer systems like TES to keep conditions constant and interpretable. Publications track the fine-tuning of TES for use in new diagnostics, combining the buffer with sensors, nanoparticles, or microfluidic devices. Instrument manufacturers rely on its non-interference with optical probes, reducing false positives and background signals. Recent work tracks the integration of TES into novel hydrogel materials for regenerative medicine, exploiting its biocompatibility for 3D tissue engineering. Researchers look for tiny differences in interaction, binding, or kinetic rates that older buffers missed, and TES enables repeatable results in the hands of postdocs and principal investigators alike.
Toxicity Research
Long-term studies demonstrate that laboratory animals tolerate TES exposure without notable systemic toxicity or organ damage, and cellular tests highlight minimal disruption to membrane integrity, metabolism, or signaling. Acute toxicity aligns with many common laboratory organic buffers, though proper precautions during weighing and transfer still matter. Regulatory reviews keep an eye out for new data, especially with the rise of extreme-scale cell culture and therapeutic protein production, but current data suggest an ample margin of safety. Wastewater research shows that TES doesn’t accumulate in aquatic life, nor does it persist in soil or groundwater compared with many industrial chemicals.
Future Prospects
Buffer chemistry marched forward as life science grew more complex and automated. Future research will likely push TES into more demanding industrial bioprocesses, particularly as researchers combine classic biological systems with microelectronics, AI-driven diagnostics, or synthetic biology. Increased interest in biocompatible scaffolds and 3D cell culture places TES at the intersection of chemistry, engineering, and medicine, and there’s growing talk about using buffers like TES to marry precision with green chemistry mandates. Advances in sustainability may pull buffer selection under tighter scrutiny, inviting innovation in recovery, recycling, and waste minimization of laboratory chemicals. If automation and robotics accelerate in clinical labs, stable, predictable, and safe buffers like TES remain crucial for data reliability and cross-border harmonization of protocols.
Everyday Workhorse in Research Labs
Scientists use a mouthful of terms in their work, and 2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid, often called TES, stands out as a staple in biological research labs. TES may sound like just another chemical, but anyone who has ever run a protein assay or tried to keep cells alive in culture knows its place on the bench. Unlike household names like table salt or sugar, TES rarely shows up outside the lab. With an eye for stability, researchers rely on it to keep conditions just right—especially the pH levels that make or break experiments with proteins, enzymes, and living cells.
Why pH Stabilization Makes a Difference
My own time working alongside molecular biologists showed me how a chemical like TES keeps everything in balance. Enzymes can be downright finicky. A minor pH shift can halt a reaction or twist a protein out of shape. Many biological processes demand a steady pH, and not every buffer can handle the job. TES comes into play because it has a “pKa” close to what many cells and enzymes crave, letting scientists mimic the body’s own environment.
Biotechnology Gets a Boost
TES finds a place in the toolkit for biotechnology, pharmaceuticals, and diagnostics. When companies develop vaccines or test new medicines, buffer solutions designed with TES provide a stable home for sensitive proteins and even help with drug formulation. If you’ve ever wondered how diagnostic tests manage to deliver consistent results, it’s because formulas like these prevent components from degrading before the test runs. Many of the newer, high-precision diagnostic tools would stall out if their mixtures couldn’t hold steady. TES doesn’t grab headlines, but its reliability forms the backbone for numerous breakthroughs, from monoclonal antibody production to platforms that screen for viruses.
Supporting Breakthroughs in Cell and Protein Science
As a former research assistant, I watched colleagues run countless experiments that depended on stable buffer systems. In protein crystallization, for example, a small pH swing can mean the difference between beautiful crystals and nothing at all. TES supports not just the proteins themselves but also entire cells during isolation or culture. The alternative—using a poorly suited buffer—leads to unpredictable results, wasted samples, and tight grant deadlines getting even tighter.
Better Alternatives, Safer Use
No buffer is perfect for every need. Some researchers look for options with less chemical interference, depending on what they’re measuring. That said, TES tends to be less likely to stick to proteins or mess with readings compared to older buffers. This leads to cleaner data, with fewer artifacts muddying the results. Safety-wise, TES should always get treated with care. Skin or eye contact, or inhalation of dust, can cause problems. Wearing gloves, lab coats, and goggles isn’t just good practice; it keeps chemists and technicians out of harm’s way, especially for those who use these chemicals every day.
Toward Greener Bench Work
The push toward sustainable laboratories has researchers weighing the impact of each chemical, including buffers. As demand for greener practices grows, companies will need to supply more transparent safety data and disposal guidelines. Simple improvements such as better labeling, clear storage protocols, and training sessions on proper handling limit risks and wasted product.
TES: The Unsung Essential
TES keeps the wheels turning in life science labs. Without dependable buffer choices, much of today’s biomedical progress would grind to a halt. The next time a groundbreaking discovery comes up, a quiet chemical like TES has likely played a role in setting the stage.
Unpacking the Chemical Structure
Scientists in lab coats love their abbreviations, but every complicated-sounding name tells a story. The chemical formula for 2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid cuts through as C6H15NO5S. That mouthful combines a backbone built from carbon, hydrogen, nitrogen, oxygen, and sulfur. With three hydroxymethyl groups, an amine, and the strong sulfonic acid group tacked together, the structure stands out. It’s this very formula that gives the compound its punch as a buffer in biology and chemistry labs.
What Makes This Buffer Special?
I remember frustration bubbling over in the lab when our pH wouldn’t stay steady during a protein purification. Not every buffer plays nice across a wide pH range, nor do all of them shrug off temperature swings and heavy salt. Using compounds like this one, with the formula C6H15NO5S, lab work calms down. The amino group and the sulfonic acid balance each other out, letting experiments run without random surprises. The molecule barely interacts with most enzymes or metal ions. So, it won’t mess with proteins the way some other acids do.
Supporting Reliable Research
Reliable buffers hold the fort in so many scientific projects. This acid’s formula comes up again and again in biochemistry journals. When you need pH adjusted to just the right sweet spot, with minimal drift, this compound delivers. One fact stands out: its low UV absorbance avoids interference with assays and measurements. Researchers get sharper data. The lack of reactivity with common biochemical players means less troubleshooting, less wasted money, and faster answers.
Addressing Supply and Safety Concerns
Lots of college chemistry labs keep this buffer on their shelves, but real-world supply isn’t always even. Demand spiked during the COVID-19 pandemic—labs worldwide scrambled to secure dependable reagents. Price hikes and availability suddenly shaped research agendas. Safety also needs to stay front and center. Though it’s not as harsh as strong mineral acids, direct contact can irritate skin and eyes. Solid lab habits mean keeping gloves on and containers sealed.
Better Solutions for the Future
To keep science moving forward, suppliers and educators can partner up. Bulk purchasing by universities smooths out bottlenecks and shaves down costs. Encouraging local production, instead of always relying on shipments from other continents, keeps shelves stocked. Training new lab workers about the strengths—and the rare weaknesses—of this buffer helps avoid wasted time during experiments. Digital tools, like lab supply inventory trackers, head off shortages before they hit. As science rolls on, reliable compounds like 2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid, formula C6H15NO5S, turn difficult protocols into routines scientists can count on.
The Chemical in Everyday Labs
2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid, often shortened to TES, shows up on reagent shelves in many labs. Folks rely on it to keep pH steady during experiments. If you’re like me, you’ve probably measured out white powder or dissolved a few grams in water before loading up an experiment. The familiarity makes it easy to treat TES like table salt, but it pays to know what you’re handling.
The Real Risks in the Lab
TES earns its spot as a buffering agent because it does not mess with reactions much. That stability doesn’t mean it’s completely without risks. Most chemical suppliers give TES a “no big deal” rating for acute toxicity. You won’t see skull-and-crossbones labels, but that doesn’t mean you can sprinkle it on your desk or inhale the dust with a shrug. Swallowing is not a free pass, either—ingestion can upset the stomach, and inhalation might irritate the nose or throat. The Safety Data Sheet (SDS) from several vendors lists “mild irritation” as the main concern.
If you spill some on your skin, it rarely does more than cause dry patches. Rinsing off with soap and water usually handles it. Splash in the eye? It stings, like most powders would. The fix: Wash your eyes, don’t rub them, and get medical help if things don’t clear up.
Why Attitude and Preparation Matter
Folks grow used to workhorse chemicals, sometimes letting their guard down. I’ve watched even skilled lab workers weigh out buffers with no gloves, thinking “It’s just another buffer.” The habit sets a bad example for those learning the ropes. Strong lab culture means folks wear gloves, eye protection, and work in places with good air flow. That mindset keeps everyone safer, even when the chemical “seems” safe.
For me, proper storage cuts down on risks, too. Keeping TES dry matters—no one wants clumps ruined by spilled water or accidental exposure. Closed containers and labeled shelves keep TES away from those who have no business handling it.
Trusting Reliable Information
The best source for chemical hazards comes from trusted organizations. PubChem, Sigma-Aldrich, and Thermo Fisher all share similar guidance: treat TES as a chemical, not a snack. Every chemical deserves respect. Even mild irritants become a problem with long exposure, neglect, or cross-contamination. Anyone who works around buffers year after year can tell you how important it is to follow protocols.
Moving Toward Better Habits
Some basic moves protect lab workers: gloves, goggles, and a dust mask for powdered handling. A well-ventilated space makes sure you don’t breathe in fine particles. Clean up spills fast, sweep and wipe down benches, and keep only what you need on your workspace. With these habits, labs dodge problems and keep accidents rare.
The fuss about safety isn’t just red tape—it stems from the health of everyone who sets foot in the lab. Good training makes a stronger lab team and sets the standard for newcomers. We care about safety because we care about each other’s well-being, and that never changes, no matter how ordinary a buffer might seem.
Why Storage Really Matters
Anyone who’s worked in a lab knows that even the best experiment can go sideways with sloppy storage habits. I once watched a whole month’s worth of careful work fall apart because a buffer compound drew moisture from the air, clumped up, and refused to dissolve. Maybe you’ve had a similar day. This is why putting effort into storing chemicals like 2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid pays dividends. This compound—better known in labs as TES—usually turns up as a white crystalline powder. What looks harmless can change its character if left in the wrong spot. It absorbs water easily, loses potency, and puts experiments at risk.
Simple Rules for Safe Storage
I’ve seen newcomers get overwhelmed by chemical storage rules. Most guidance boils down to common sense: treat everything as if it's an investment. For TES, this means keeping it dry and cool. Avoid the drama by sealing the original bottle right after measuring. If the cap cracks or starts to warp, switch containers. Leaving any powder exposed, even for a few hours, bumps up the chance it will clump or change. Some labs use desiccators for this very reason—they pull moisture from the air and help powders last longer. Don’t stash the bottle near windows, radiators, or other hot spots. Light and heat speed up the breakdown of sensitive chemicals, making buffers like TES useless right when you need them.
The Value of a Dedicated Space
Crowded lab benches put bottles in harm’s way. One spill can wipe out a whole department’s supply. I suggest giving TES its own labeled section, away from strong acids, bases, and anything volatile. You might not think a little bottle would matter, but more than one lab has learned the hard way that chemical cross-contamination ruins results—and even equipment. If possible, keep it with other buffers and not alongside solvents. This helps technicians and students grab the right bottle and track inventory without mistakes. Avoid moving the compound from its home unless absolutely necessary.
Keeping the Label Honest
People laugh about difficult chemical names, but a worn or faded label spells disaster. I once opened a mystery bottle only to realize everyone had guessed, and no one had written the date or owner on the label. Use permanent ink and note the date each time the seal breaks. If you have a batch that’s been open for a few months, think about replacing it, especially in a high-humidity area. A well-maintained label keeps colleagues safe and experiments reliable.
Building Good Habits Together
At its core, storing TES well comes down to shared discipline. If one person skips sealing the bottle, someone else pays for it down the line. Lab meetings work well for sharing tips and flagging when supplies look off. Keep an eye out for powder that’s become grainy or sticky—signs that moisture got in. Ask peers to double-check, especially if students are learning the ropes. Labs that approach chemical storage as a team sport enjoy better experiments and fewer costly surprises. With careful attention, that white bottle of TES stays effective, ensuring research isn’t derailed by avoidable mistakes.
Understanding Why Chemists Care About Buffer Ranges
Anyone who’s ever fiddled around in a wet lab knows that reliable pH buffering is like solid boots in muddy fields—absolutely crucial if you want meaningful results. One buffer that comes up more than you’d think is 2-Tris(Hydroxymethyl)methyl Amino Ethane-1-sulphonic Acid, but most folks just call it TAPS. TAPS jumps into action whenever a project demands maintaining a specific pH, particularly if the target sits between 7.7 and 9.1. That’s not just trivia; it’s the actual pH zone where TAPS delivers its steady results, and countless researchers have put that number to the test in everything from protein work to electrophoresis.
Why a pH Range Matters
It’s tempting to gloss over the details, but the reason this buffer keeps cropping up is because experiments get twitchy if pH starts swinging around. I remember setting up a protein expression run, back when every penny in the grant counted. The protocol called for a buffer at pH 8.5. Plenty of choices, but TAPS gave a real sweet spot—not too acidic, not drifting into the basic danger zone where proteins can denature. The stability of TAPS buffers in that 7.7 to 9.1 window isn’t just textbook knowledge. You see it on lab notes, in data repeatability, and especially in avoided headaches when troubleshooting set-ups. In practical terms, I’ve watched less stable buffers throw off results, slow down troubleshooting, and even wreck a few weekends—something every bench scientist wants to avoid.
Fact-Checking the Chemistry
This buffer doesn’t just win on experience alone. The pKa of TAPS—8.4 at 25°C—has stood up in decades of published work. It lands TAPS right in the range for DNA/RNA research or protein purification where even fractional pH shifts can change results. Earnest Good and his colleagues catalogued TAPS among the zwitterionic “Good’s buffers” lineup, which all focus on minimal reactive side effects and real stability under experimental heat or cold. Those aren’t marketing claims; they’re results measured by scientists for scientists.
Researchers reach for TAPS in molecular biology, especially during polyacrylamide gel electrophoresis, because it lets charged molecules line up and travel predictably through gels. Inside that crucial 7.7 to 9.1 pH range, TAPS works right alongside other classic “Good’s buffers,” but with a bit more strength in the basic direction. Anyone doing even basic protein or nucleic acid work has good reason to want that reliability when planning weeks-long experiments.
What Happens Outside the Range?
Stepping out of the recommended pH window brings headaches that can upend months of planning. Too far above or below that range, and the buffer loses its fighting power—pH drifts, samples degrade, and those careful plans end up in the recycling bin. I’ve had colleagues try to stretch buffers for obscure protocols, only to retest everything after noisy or unreadable results. A mismatched buffer undermines downstream analysis, wasting both samples and budget.
Better Buffer Choices Start With Honest Knowledge
Picking TAPS for work in that 7.7 to 9.1 range just makes sense, and not just because the numbers line up in handbooks. It means experiments get backed by a buffer proven to hold together under real conditions. We can’t ignore the lessons learned on real benches with work that actually delivers. Staying loyal to those proven ranges, supported by decades of chemical data and practical use, helps experiments succeed and keeps everyone’s schedule a little less chaotic.